
Predictive clinicopathological characteristics affecting sentinel lymph node metastasis in early breast cancer patients
Introduction
Breast cancer is now the most common cancer in Chinese women, and the leading cause of cancer death in women younger than 45 years (1,2). Axillary lymph node status is one of the most valuable predictors for the survival of breast cancer patients (3,4). Axillary lymph node dissection (ALND) has been accepted as the standard surgical treatment for breast cancer patients, and made great sense in assessment of lymph nodes status and regional tumor control (5). However, sentinel lymph node biopsy (SLNB), as a minimally invasive surgery, has become the alternative treatment of conventional ALND, especially for the patients with clinically negative axillary lymph nodes (6-8). Although SLNB has some advantages such as better cosmetic results, less limb complications and more rapid intraoperative diagnosis, it is also an invasive procedure with complications. Moreover, the reported incidence of sentinel lymph node (SLN) metastasis varies from 22–40% (9-11), which means nearly 60–78% patients suffered from unnecessary invasive axilla surgery. It becomes important that whether we could make a predictive model to determine appropriate patients who might avoid SLNB and unnecessary surgery. Since the predictive factors affecting the SLN status have not been clarified yet, we performed this study to identify the clinicopathological predictors of SLN involvement.
Methods
Ethics statement
This study was approved by the Ethics Committee of the Peking Union Medical College Hospital, Chinese Academy of Medical Sciences.
Patients and clinicopathological characteristics
Our retrospective study analyzed consecutive patients who were diagnosed as early invasive breast cancer without clinically detected axillary lymph nodes and underwent breast surgery and SLNB in Department of Breast Surgery, Peking Union Medical College Hospital (PUMCH), between January 2014 and December 2016. In total, 324 patients were finally enrolled in this study. All patients’ formalin-fixed paraffin-embedded (FFPE) pathological sections were reviewed to confirm the diagnosis, and the clinicopathological characteristics were collected thoroughly.
SLNB
SLNB was performed through both the methylene blue dye and the indocyanine green (ICG) (12,13) injection to the subareolar zone, minimal 10 minutes before the biopsy. Blued nodes were detected and excised with their lymphatic vessel, and double checked with fluorescence device. The SLN was diagnosed by the intraoperative frozen pathological section and was finally determined by the FFPE examination. ALND was the necessary procedure in case of detection of SLN metastasis.
Statistic analysis
As for detecting predictors of SLN metastasis, the quantitative variables were compared with t-test and the categorical variables were compared with chi-square tests or Fisher’s exact test. Then multivariate logistic regression analysis was performed to test the independent predictors for all related clinicopathological characteristics from the univariate analysis (14,15). The significance threshold was set at P<0.05. Meanwhile, the odds ratio (OR) and 95% confidence intervals (CI) were also counted. SPSS software, version 20.0 (SPSS, Inc. Chicago, IL, USA) was used for all of the statistical analyses.
Results
Descriptive information of the study cohort
A total of 324 breast cancer patients underwent breast surgery and SLNB in our study. All patients were female, and all the clinicopathological characteristics were showed (Table 1). As for the SLNB, totally 1,334 SLNs were excised, with the average number 4.12±2.82. Sixty-six of 324 patients had positive SLN and received following ALND, which indicated our incidence of SLN metastasis was 20.4%. Of these 66 patients, 61 (92.4%) had macro-metastasis, 3 (4.6%) had micro-metastasis and 2 (3.0%) had isolated tumor cell (ITC).
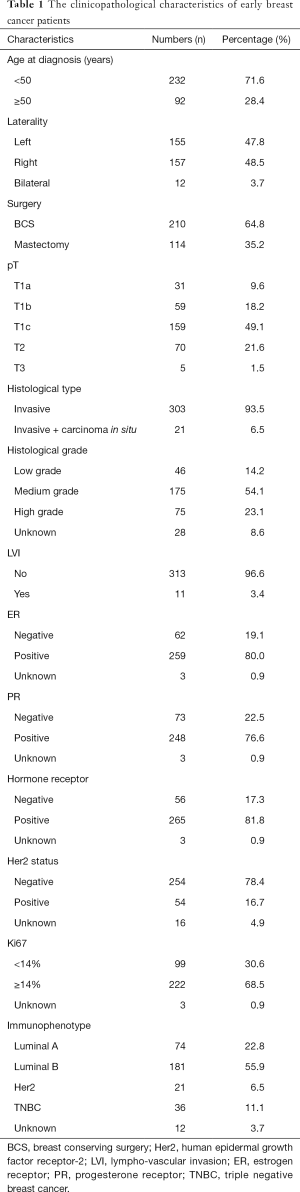
Full table
Univariate analysis between clinicopathological characteristics and SLN status
According to the univariate analysis, our study found that tumor size, pT stage, lympho-vascular invasion (LVI), estrogen receptor (ER) status, hormone receptor (HR) status, and triple negative breast cancer (TNBC) were associated with SLN metastasis. Compared to patients with negative SLN, patients with positive SLN had bigger tumor size (2.017±1.236 vs. 1.646±1.114, P=0.019), higher pT stage (P=0.027), more LVI (7.6% vs. 2.3%, P=0.036), more ER positive cancer (90.9% vs. 77.1%, P=0.041), more HR positive cancer (92.4% vs. 79.0%, P=0.040), and less TNBC (3.0% vs. 13.2%, P=0.019) (Table 2).
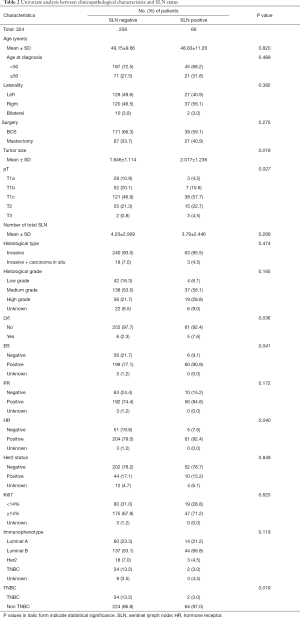
Full table
Multivariate logistic regression analysis for the predictors of SLN metastasis
All the related clinicopathological characteristics from the univariate analysis (P<0.20), including tumor size, pT stage, histological grade, LVI, ER status, progesterone receptor (PR) status, HR status, immunophenotype and TNBC status, were calculated in the multivariate logistic regression analysis by forward stepwise method. Finally, histological grade, pT stage and TNBC status were the independent predictive factors (Table 3).

Full table
Discussion
Since SLNB could appropriately assess axillary lymph node status, it has been demonstrated as the technical standard surgical procedure instead of ALND for the breast cancer patients with clinically negative axilla (6,7,16). Even though SLNB has fewer postoperative morbidity than that of ALND, it is reported that SLNB accounted for about 7–15% incidence of upper limb lymphedema, or 8–16% incidence of sensory loss or pain (17-19). Moreover, preoperative prediction of SLN status with various clinicopathological characteristics might avoid the unnecessary SLNB in selected patients, which could consequently save more medical resources.
In our study, we used dual tracer method with methylene blue dye and ICG, and identified the average of 4.12 SLNs per participants, which demonstrated that SLNB with blue dye and fluorescence is a reliable and effective surgical technique. What’s more, the detection rate of positive SLN was 20.4% in the present study, which was lower than that in previous ones (9-11). This discordance may be a result of more pT1 stage patients, which comprised 76.9% in our study and higher than about 50–60% in other studies.
Therefore, it brings the tumor size to us as the first and most common predictor for SLN metastasis. Previous literatures that the possibility of SLN or/and axillary lymph node involvement increased with the accretion of tumor (20-22). In the present study, we figured out the same opinion, which is that pT stage is the independent predictive factor of SLN metastasis in patients with early breast cancer (adjusted OR =2.169, 95% CI, 1.065–4.417, P=0.033), according to both univariate and multivariate analyses.
Secondly, in previous studies, histological grade was shown an important predictor, which was even more valuable than tumor size (23-25). Similarly, we found that histological grade had no significant difference between these two groups by univariate analysis (P=0.165), but it was demonstrated as the predictive factors of SLN positivity by multivariate analysis (adjusted OR =1.415, 95% CI, 1.004–1.996, P=0.048).
In addition, the role of TNBC that played in the SLN metastasis has been controversial. As we all know, TNBC compromised about 10–20% of all breast cancers, and exhibited more aggressive clinical behavior, higher metastatic potential and poorer prognosis when compared to other immunophenotype (26-28). However, the final result in our study figured out that TNBC was a negative predictor of SLN involvement, with adjusted OR as 0.506 (95% CI, 0.307–0.835, P=0.008). This conclusion was supported by many scholars who have identified that TNBC were associated with low incidence of axillary lymph node metastasis, and suggested that the poor biological features of TNBC might be not associated with lymphatic spread (29-31).
Besides these three predictive clinicopathological characteristics, young age at diagnosis, the presence of LVI, high Ki67 values, overexpression of Her-2 and other factors had been selected as the predictor affecting SLN metastasis in early breast cancer patients (32-37).
Our study had several limitations. Firstly, this was a retrospective study with relatively small sample size in single institution. We should recruit more participants for a new randomized, double-blind, multicenter clinical trial to verify the truth in the near future. Secondly, not all the detailed clinicopathological characteristics were collected through the patients’ medical record which was due to the limitation of data storage system. More details should be reviewed if possible. Last but not least, although exact pathological evaluation of all tumor and SLN FFPE specimens was reviewed by experienced breast pathologists, there were still some time or technical limitations in detection of more biomarkers for all pathological sections, such as EGFR, CK 5/6 or androgen receptors, etc. If we could get more about these details, it maybe provides us more interesting and useful information.
Conclusions
In conclusion, our study demonstrated that pT stage and histological grade provided positive, and TNBC provided negative prediction about SLN metastasis in early stage breast cancer patients with clinically negative axillary lymph nodes. In spite of the limitations, the results are useful in clinical practice. We should run more clinical trials to optimize the precise predictive factors or models, in order that more patients could not suffer from ALND, or even SLNB in the future.
Acknowledgments
We would like to thank all of the patients for their devotion of medical records in our study.
Funding: This work was supported by the Twelfth Five Year Key Programs for Science and Technology Development of China (Grant No. 2014BAI028B03).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Peking Union Medical College Hospital, Chinese Academy of Medical Sciences. Since this is a retrospective study without any intervention or treatment to patients, the Ethics Committee Board just approved it without giving an ID. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983;52:1551-7. [Crossref] [PubMed]
- . Consensus statement: treatment of early-stage breast cancer. National Institutes of Health Consensus Development Panel. J Natl Cancer Inst Monogr 1992;1-5. [PubMed]
- Jatoi I, Hilsenbeck SG, Clark GM, et al. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol 1999;17:2334-40. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol 2006;7:983-90. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Rao R, Euhus D, Mayo HG, et al. Axillary node interventions in breast cancer: a systematic review. JAMA 2013;310:1385-94. [Crossref] [PubMed]
- Chua B, Ung O, Taylor R, et al. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg 2001;71:723-8. [Crossref] [PubMed]
- Nandu VV, Chaudhari MS. Efficacy of Sentinel Lymph Node Biopsy in Detecting Axillary Metastasis in Breast Cancer Using Methylene Blue. Indian J Surg Oncol 2017;8:109-12. [Crossref] [PubMed]
- Jiao D, Qiao J, Lu Z, et al. Analysis of predictive factors affecting sentinel lymph node status in early breast cancer patients. Zhonghua Zhong Liu Za Zhi 2014;36:198-201. [PubMed]
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. [Crossref] [PubMed]
- Zhang X, Li Y, Zhou Y, et al. Diagnostic Performance of Indocyanine Green-Guided Sentinel Lymph Node Biopsy in Breast Cancer: A Meta-Analysis. PLoS One 2016;11:e0155597 [Crossref] [PubMed]
- Xu L, Kim Y, Spolverato G, et al. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surg Nutr 2016;5:43-52. [PubMed]
- Bodzin AS. Hepatocellular carcinoma (HCC) recurrence and what to do when it happens. Hepatobiliary Surg Nutr 2016;5:503-5. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 2006;95:279-93. [Crossref] [PubMed]
- Burak WE, Hollenbeck ST, Zervos EE, et al. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg 2002;183:23-7. [Crossref] [PubMed]
- Kootstra JJ, Dijkstra PU, Rietman H, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat 2013;139:125-34. [Crossref] [PubMed]
- Wang H, Wang J, Gao JD, et al. Analysis of influencing factors to metastasis in sentinel lymph nodes and non-sentinel lymph nodes in breast cancer. Zhonghua Zhong Liu Za Zhi 2013;35:769-72. [PubMed]
- Fujii T, Yajima R, Tatsuki H, et al. Significance of lymphatic invasion combined with size of primary tumor for predicting sentinel lymph node metastasis in patients with breast cancer. Anticancer Res 2015;35:3581-4. [PubMed]
- Viale G, Zurrida S, Maiorano E, et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 2005;103:492-500. [Crossref] [PubMed]
- Henson DE, Ries L, Freedman LS, et al. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index. Cancer 1991;68:2142-9. [Crossref] [PubMed]
- Sundquist M, Thorstenson S, Brudin L, et al. Applying the Nottingham Prognostic Index to a Swedish breast cancer population. South East Swedish Breast Cancer Study Group. Breast Cancer Res Treat 1999;53:1-8. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Montagna E, Maisonneuve P, Rotmensz N, et al. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Cancer 2013;13:31-9. [Crossref] [PubMed]
- Gangi A, Chung A, Mirocha J, et al. Breast-conserving therapy for triple-negative breast cancer. JAMA Surg 2014;149:252-8. [Crossref] [PubMed]
- Crabb SJ, Cheang MC, Leung S, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer 2008;8:249-56. [Crossref] [PubMed]
- Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 2009;16:2705-10. [Crossref] [PubMed]
- Reyal F, Rouzier R, Depont-Hazelzet B, et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One 2011;6:e20297 [Crossref] [PubMed]
- Lyman GH, Somerfield MR, Giuliano AE. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: 2016 American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract 2017;13:196-8. [Crossref] [PubMed]
- Shokouh TZ, Ezatollah A, Barand P. Interrelationships Between Ki67, HER2/neu, p53, ER, and PR Status and Their Associations With Tumor Grade and Lymph Node Involvement in Breast Carcinoma Subtypes: Retrospective-Observational Analytical Study. Medicine (Baltimore) 2015;94:e1359 [Crossref] [PubMed]
- Yoshihara E, Smeets A, Laenen A, et al. Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast 2013;22:357-61. [Crossref] [PubMed]
- Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174-83. [Crossref] [PubMed]
- Dihge L, Bendahl PO, Rydén L. Nomograms for preoperative prediction of axillary nodal status in breast cancer. Br J Surg 2017;104:1494-505. [Crossref] [PubMed]
- Thangarajah F, Malter W, Hamacher S, et al. Predictors of sentinel lymph node metastases in breast cancer-radioactivity and Ki-67. Breast 2016;30:87-91. [Crossref] [PubMed]


