
Nivolumab for chemorefractory oesophageal squamous cell carcinoma
Nivolumab for chemorefractory oesophageal squamous cell carcinoma (OSCC)
Although immunotherapy has radically changed the treatment paradigm for a variety of tumours including melanoma, non-small cell lung cancer and renal cell carcinoma, until now it has been less successful in tumours of the gastrointestinal tract. In a recent report in Lancet Oncology, Kudo et al. described the results of the first trial assessing immune checkpoint blockade in OSCC patients (1). The study enrolled 65 Japanese patients with platinum and taxane refractory OSCC in a standard open-label phase II design, the primary endpoint of which was centrally assessed objective response rate (ORR). OSCC patients treated with nivolumab on the trial had an ORR of 17% [95% confidence interval (CI), 10–28%] by central radiological review. However, a further proportion of patients appeared to benefit from anti-PD-1 therapy but did not reach RECIST criteria for response; a reduction in overall tumour burden was observed in 45% of participants by the trial investigators. In keeping with many studies of immunotherapy, median progression-free survival (PFS) did not appear to be substantially improved by nivolumab (median PFS, 1.5 months; 95% CI, 1.4–2.8 months), however, median overall survival (OS) was promising for a chemorefractory patient population at 10.8 months (95% CI, 7.4–13.3 months). These results are potentially important because if validated in a randomised controlled trial, nivolumab could provide a new treatment option for advanced OSCC, a cancer for which very little evidence is available to guide clinical management.
Historically, few trials have focused on treatment for metastatic OSCC patients, and most treatments for OSCC are based on data derived from trials of gastric or gastroesophageal adenocarcinoma. Phase III randomised trials support the use of single agent taxane or irinotecan chemotherapy in the second-line setting; the absolute benefit associated with salvage chemotherapy is approximately 6 weeks, with median OS of less than 6 months expected (2-4). Two small studies of combination chemotherapy for chemorefractory OSCC suggest higher response rates of 23–30% for taxane-gemcitabine or taxane-fluoropyrimidine combinations; however, these regimens have not been validated in larger, randomised trials (5,6). Considering the generally poor radiological response rates associated with single agent salvage chemotherapy, in the trial reported by Kudo and colleagues, it is impressive that nivolumab was able to achieve a radiological response rate of 17% in a heavily pretreated population refractory to both cisplatin and taxane chemotherapy. Furthermore, the median OS associated with nivolumab therapy appears to be longer than that seen with taxanes in the in second-line setting. Importantly, in a patient group which may have co-morbidities associated with tobacco and alcohol use, the toxicity profile of nivolumab was not different from what is already known. Although standard immunotherapy-related side effects were observed (i.e., diarrhea, rash, abnormal hepatic function and fatigue), serious adverse events were uncommon (n=11, 17%) and relatively few patients (n=7, 11%) discontinued treatment due to toxicity. Prior to the publication of Kudo and colleagues in Lancet Oncology, few data on immune checkpoint blockade (i.e., PD-1, PD-L1 and CTLA-4 inhibitors) were available relating specifically to OSCC. The KEYNOTE 028 study, which was reported in abstract form only, assessed the efficacy of pembrolizumab in PD-L1 positive oesophageal cancer (of both adenocarcinoma and OSCC histology) (7). In KEYNOTE 028, 17 patients with PD-L1 positive OSCC were treated with pembrolizumab, of whom 29% (n=5) had an objective radiological response. In particular, although emerging data supports the routine use of nivolumab in chemorefractory gastric and gastroesophageal adenocarcinoma, the biological distinctions between oesophageal adenocarcinoma and OSCC mean that results from gastroesophageal adenocarcinoma trials cannot be readily extrapolated to patients with OSCC (8). The work of The Cancer Genome Atlas (TCGA) demonstrates that whereas from an oncogenomic perspective oesophageal adenocarcinoma is strongly reminiscent of chromosomally unstable gastric cancer, OSCC resembles squamous cell carcinoma of other organs, including head and neck squamous cell carcinoma (HNSCC) and squamous non-small cell lung cancer (SqNSCLC) (9).
Because aetiologically and pathologically OSCC is more closely molecularly related to HNSCC and sqNSCLC than gastric or gastroesophageal adenocarcinoma, the results of immune checkpoint blockade in HNSCC and SqNSCLC may be of relevance for development of anti-PD-1 therapy in OSCC. In the CheckMate 141 trial which evaluated nivolumab vs. methotrexate, docetaxel or cetuximab in a unselected platinum-refractory HNSCC population, the ORR associated with nivolumab treatment was 13.3%, and nivolumab significantly increased median overall and 1-year survival compared to standard therapy [median OS, 7.5 vs. 5.1 months; hazard ratio (HR), 0.70; P=0.01 and 36.6% vs. 16.0%, respectively] (10). In a subgroup analysis of Checkmate 141, median OS was statistically significantly improved only for HNSCC patients who were PD-L1 positive in ≥1% of tumour cells. Although results of nivolumab efficacy in OSCC according to PD-L1 expression are not reported by Kudo et al., the PD-L1 expression associated survival benefit demonstrated in HNSCC for nivolumab might suggest that evaluation of PD-L1 status in OSCC could select a subgroup of patients with increased benefit from PD-1 inhibition. However conversely, results in sqNSCLC trials did not support a predictive role for tumoural PD-L1 expression (at the 1–10% level) for nivolumab therapy, but did for pembrolizumab (at 50% PD-L1 positivity using a different assay) (11,12). Therefore, it is evident that each tumour site, even if molecularly similar, must be evaluated independently for the interaction between PD-L1 expression, which may be antibody dependent, and efficacy of immune checkpoint blockade. Interestingly, in the largest retrospective series evaluating the prognostic role of PD-L1 expression in patients with surgically resected OSCC, PD-L1 expression in ≥5% of immune infiltrating cells but not tumour cells was positively prognostic for OS (13). Therefore, in future trials, evaluation of PD-L1 status on immune infiltrating cells rather than tumour cells may be of more value for OSCC patients.
Notably, the results presented by Kudo et al. were achieved in a clinical trial which recruited only Japanese patients. Therefore, whether these results are generalizable to non-Asian patients is a relevant question. However, the TCGA assessment of oesophageal cancer included a global patient population and found no significant differences in genomic signatures between Eastern and non-Asian patients, although some regional trends were noted (9). Moreover, although there is evidence suggesting that the immune microenvironment varies between Asian and non-Asian gastric cancer patients, there are no data suggesting the same in the OSCC setting (14). Finally, in an early report of the efficacy of pembrolizumab in PD-L1 positive gastric cancer, there was no difference in efficacy between Asian and non-Asian patients, nor has there been any suggestion of differential efficacy of anti-PD-1 in Asian NSCLC patients (12,15). Therefore, future trials of anti-PD-1 therapy for OSCC might reasonably combine patients from different geographic regions worldwide. Ongoing and upcoming trials examining immunotherapy agents, either as a single agent or in various combination strategies, in the OSCC setting are summarised in Table 1.
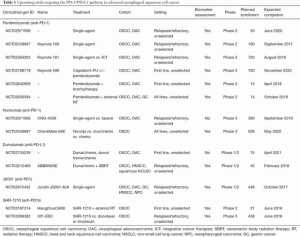
Full table
In conclusion, the results presented by Kudo and colleagues are encouraging for patients with OSCC. By focusing their analysis on OSCC patients, they have set an important benchmark in separating the two oesophageal cancer histologies. In doing so, it should be possible to derive stronger conclusion from trials and ultimately provide benefit to patients. Identification and validation of predictive biomarkers to establish which patients are likely to benefit from immune checkpoint inhibitors remains a priority in all cancers, including OSCC. Equally, understanding the mechanisms of resistance to anti-PD-1 therapy in OSCC is essential, as the majority of patients do not benefit from immune checkpoint blockade. This knowledge will be important in designing future trials, in particular combination strategies.
Acknowledgments
Funding: M Chénard-Poirier and EC Smyth acknowledge the funding support of the National Institute for Health Research Royal Marsden Institute of Cancer Research Biomedical Research Centre (NIHR RM/ICR BRC).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, The First Affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: EC Smyth declares honoraria for advisory role from Five Prime Therapeutics, Bristol Meier-Squibb, Gritstone Oncology and Servier. M Chénard-Poirier has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Lee MY, Jung KS, Kim HS, et al. Weekly docetaxel and gemcitabine in previously treated metastatic esophageal squamous cell carcinoma. World J Gastroenterol 2015;21:4268-74. [Crossref] [PubMed]
- Li X, Lin W, Wang H, et al. Phase II trial of second-line chemotherapy with docetaxel and capecitabine in advanced esophageal squamous cell carcinoma. Med Oncol 2013;30:746. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol 2016;34:7. [Crossref]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017;35:2. [Crossref]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Zhang W, Pang Q, Zhang X, et al. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci 2017;108:590-7. [Crossref] [PubMed]
- Lin SJ, Gagnon-Bartsch JA, Tan IB, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015;64:1721-31. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]


