A concise review of current guidelines for the clinical management of hepatocellular carcinoma in Asia
Introduction
Hepatocellular carcinoma (HCC) has been the fifth most common malignancy worldwide, with over 500,000 new cases every year, and it serves as the third most common cause of tumor-related mortality globally (1-4). Furthermore, it is particularly endemic in Asia, accounting for around 80% of new cases around the world (5,6). In the last 20 years, numerous researches have explored the clinical management of HCC (7-10). However, the overall prognosis are still unsatisfactory, especially seen in Asian countries. Guidelines are defined as “systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances” (11). The full performance of guidelines could accomplish the following aims: (I) up-to-date for individualized decision-making algorithms by clinicians; (II) promotion of the healthcare quality; (III) better resource apportion by superior administrations (9). Since the Korean Liver Cancer Study Group and National Cancer Center jointly issued the first HCC guideline in Asia in the year of 2003, various Asian guidelines have been published or updated till now. Despite of this, controversies in certain aspects of HCC management evidently existed. Thus, the aim of the present study is to incorporate new evidence on the management of HCC by assessing and comparing the main Asian guidelines.
Methods
Study identification
For this review, electronic databases of MEDLINE (via PubMed), the Chinese SinoMed (http://www.sinomed.ac.cn/zh/) and the Japanese CiNii (http://ci.nii.ac.jp/) were systematically searched from the initiation of the databases to September, 2017. No language restriction was applied to the search strategy. Search terms (medical subject headings or keywords) included: “hepatocellular carcinoma”, “guidelines/practice guidelines”, “consensus”, “liver cancer”, and “liver carcinoma”. Inclusion criteria were as follows: (I) influence, records were drafted with the support of government or academic/medical societies, then cited by subsequent guidelines or other publications on the management of HCC; (II) multifaceted, records at least covered the contents of diagnosis and treatment of HCC; (III) drafting body, records were created and endorsed by the Asian countries or academic organizations; and (IV) publication form, records should be issued in the form of clinical guidelines or expert consensus. A study meeting all four criteria was regarded eligible to be included. Furthermore, reference lists of guidelines were searched manually for potential target articles. In the case of one guideline having different updated versions, only the most recent version was included.
Guidelines appraisal
As a highly useful evaluation tool for guidelines, the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument was utilized for the quality assessment of guidelines by examining six domains covering 23 key items (six domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, editorial independence) (12). Each item in the AGREE II was separately graded by three reviewers (H Tang, Y Huang, and C Li) on a 7-point scale (1, strongly disagree to 7, strongly agree) (12).
Each domain score will be calculated by summing up all the scores of the individual items in a domain and by scaling the total as a percentage of the maximum possible score for that domain (12). The formula is:
Minimum possible score refers to number of items multiplied by number of reviewers, multiplied by 1 (strongly disagree); minimum possible score = (number of items) × (number of reviewers) × (1, strongly disagree). Maximum possible score refers to number of items multiplied by number of reviewers, multiplied by 7 (strongly agree); minimum possible score = (number of items) × (number of reviewers) × (7, strongly agree).
To ensure accuracy and minimize bias of guidelines appraisal, the intra-class correlation coefficient was introduced for the assessment of between-reviewer agreement (the two-way random model, by SPSS) (13).
Results
Current main guidelines of HCC in Asia
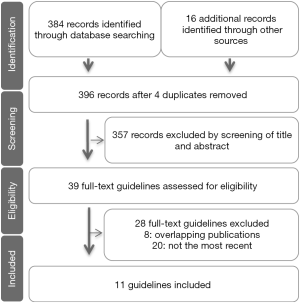
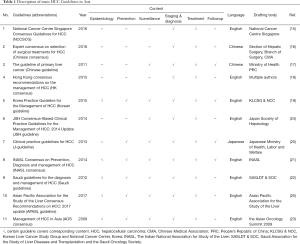
Figure 1 showed the process of study selection. A total of 396 references were produced using the outlined search strategy. Finally, 11 guidelines were identified and then included for comparison (14-24). Ten of the total 11 guidelines were published in the academic journals, and one was in a book (Japanese clinical practice guidelines for hepatocellular carcinoma, J-HCC); the majority (8 of 11) was published in English, two were in Chinese, and one was in Japanese. The guidelines were drafted in Singapore (one guideline), China (three guidelines, including one in Hong Kong, SAR), Republic of Korea (one guideline), Japan (two guidelines), India (one guideline), or Saudi Arabia (one guideline) or that were multination (two guidelines) between 2009 and 2017. Guideline characteristics were summarized in Table 1.
Full table
Results of guidelines appraisal
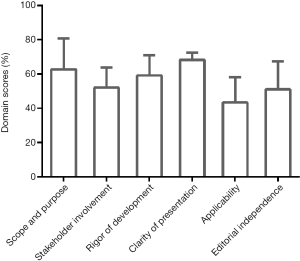
Results of guideline appraisal by AGREE II were illustrated in Table 2 and Figure 2. In Table 2, domain score and overall assessment for each guideline were demonstrated. Most guidelines obtained average scores over 50%. From an overall perspective, Korea practice guideline for the management of HCC (Korean Guideline) that covered all 6 aspects (epidemiology, prevention, surveillance, staging & diagnosis, treatment, and follow-up) featured the highest score. At the same time, five guidelines being scored less than 6 in overall assessment might be recommended with modifications according to the AGREE II. By calculating the mean score of each domain for the included guidelines, comparatively high mean scores were granted for the domains of ‘Scope and purpose’ (mean 62.88, standard deviation 18.01) and ‘Clarity of presentation’ (68.37, 4.15), and low score for the domain of ‘Applicability’ (43.43, 14.71), which was shown in Figure 2. The intra-class correlation coefficients range from 0.61 to 0.83, indicating fair-to-good agreements between reviewers.

Full table
Comparison of the guidelines
Based on the appraisal results of AGREE II, six guidelines that were rigorously drafted and developed achieved no less than 6 score in overall assessment and could be recommended without modifications. Then, on this occasion, a concise comparison of the guidelines was then conducted mainly focusing on three sections concerning HCC clinical management (risk factors and surveillance, diagnosis, and treatment). Meanwhile, special focus was relatively placed on the guidelines achieving higher overall score or domain score.
Risk factors and surveillance
Totally, 8 of the total 11 guidelines clearly involved the content of surveillance (Table 1). Although most of the content about the risk factor and surveillance are commensurate, variances still existed among guidelines.
Risk factors for HCC can be divided into two aspects: cirrhosis-related and non-cirrhosis-related. The former consists of hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, alcoholic cirrhosis, genetic causes, nonalcoholic steatohepatitis, stage four primary biliary cirrhosis, alpha one antitrypsin deficiency, and other causes of cirrhosis; the latter covers HBV carrier with family history of HCC, Asian population with older ages (males ≥40 years and females ≥40 years), and Africa/North American Blacks with Hepatitis B (25). Hepatitis B serves as the leading cause of HCC in China, and hepatitis C in Japan (26,27). Cirrhosis that could be caused by various etiologies is the most powerful HCC predictor (28,29).
Regarding screening method, the combined use of ultrasonography (US) and alpha-fetoprotein (AFP) are the most common and effective measure for HCC detection around the world (30,31). Of note, increased AFP level might be found in less than 20% of patients with early stage HCC (32-34). On this occasion, AFP was ruled out (11,35,36). However, certain Asian experts still believed AFP is a useful surveillance tool (37) and the role in combination with US, which could contribute to the early detection of HCC (38). Among currently included guidelines, eight guidelines involved the use of AFP for surveillance (or screening). Six guidelines recommended US in combination with AFP (16,18,19,22-24), while two guidelines suggested US alone (20,21).
Yet, controversies remain over the use of other biomarkers, such as lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) or des-gamma-carboxy prothrombin (DCP). So far, their role in surveillance requires further validation, and only Japanese guidelines included DCP and AFP in their recommendation of screening (19,39-41).
Although there is still a lack of definite consensus on the most recommended surveillance interval to take, the general interval of surveillance is 6 to 12 months among worldwide guidelines. In Asian perspective, performing surveillance every 6 months instead of annually was uniformly recommended. Furthermore, as to high risk patients, a close surveillance interval is preferred. In Japan Society of Hepatology-HCC Guideline (JSH Guideline), a 3-month surveillance interval is essentially performed for patients at super high risk for HCC (i.e., those with cirrhosis of HBV or HCV) (23,42). For HBV patients, 6-month surveillance is demonstrated to be superior to 12-month one with regards to the detection of early HCC and overall survival (43).
In Japanese guidelines, population who are associated with high risk of HCC and need surveillance are classified as super high risk population and high risk population (19,23,42). Super high-risk population covers: (I) hepatitis B related liver cirrhosis; (II) hepatitis C associated cirrhosis. Surveillance plan for those is US examination and tumor marker (AFP/PIVKA-II/AFP-L3) measurements every three to four months, or dynamic computed tomography (CT)/magnetic resonance imaging (MRI) every 6 to 12 months for cirrhosis and obesity patients with difficulty in US evaluation. High risk population includes: (I) chronic hepatitis B; (II) chronic hepatitis C; (III) liver cirrhosis (non-HBV or HCV related). The recommended surveillance is US examination and tumor marker measurements semiannually.
Diagnosis
Although variations exist among these guidelines, the final diagnosis of HCC relied on imaging techniques or biopsy.
Imaging techniques feature significant value in clinical management of HCC, including screening and surveillance, diagnosis, staging, and follow-up. Among common imaging techniques, US carries a vital role in the HCC detection for its cost-effectiveness and wide availability. However, it has the limitation of relatively low sensitivity for the identification of smaller nodules in a cirrhotic liver (44). Of note, the Asian Pacific Association for the Study of the Liver consensus recommendations (APASL guideline) implies that contrast enhanced US could be of equal sensitivity in HCC diagnosis in comparison with CT and MRI (45). Moreover, contrast enhanced US brings the advantage of real-time scanning throughout the whole arterial phase, with a contrast agent which is purely intravascular and can be safely administered in patients with renal failure. On this occasion, although contrast enhanced US was ruled out in certain western guidelines (35,46), it is still in use of certain diagnostic algorithm issued by some expert panel. Dynamic CT or dynamic MRI is suggested as the first-line diagnostic tools for HCC when a screening examination produces a suspected finding. Hallmark of a tumor during dynamic CT or dynamic MRI scan (arterial hyperenhancement with ‘‘washout’’ in the portal venous or delayed phase), regardless of tumor size, will suffice for a diagnosis of HCC, and obviates the need for biopsy. Moreover, MRI might perform better than CT in distinguishing HCC lesions from cirrhotic nodules in cirrhotic livers.
Serum AFP concentration serves as a helpful diagnostic tool in case that imaging technology fails to suffice for a definite diagnosis of HCC. It is generally accepted that serum AFP at least 400 mcg/L in a high-risk population would be of diagnostic value for HCC (47).
Currently, biopsy is limitedly utilized in the diagnosis of HCC for the reduction of potential bleeding or needle-track tumor seeding (approximately 2.7%) (48). Particularly, if patients present with typical imaging and are candidates for curatively intended surgery, then biopsy should be avoided. Moreover, it is emphasized that a negative biopsy result could not rule out HCC when the nodule size is increasing (25). Biopsy could only be the last choice for nodule diagnosis in cirrhotic patients who lack classical imaging presentation. But it’s still recommended for nodules in non-cirrhotic livers.
Furthermore, we analyzed the diagnostic algorithms in each guideline and found that there are two main types of diagnostic pathways on the detection of a nodule or mass by US: size-based pathway, and non-size-based pathway. The former was proposed by five guidelines (14,16,18,20,21), and the latter by 4 (19,22-24).
In regard to size-based pathway, diagnostic algorithms will be initiated with tumor size (whether size exceeding 1 cm). Small HCC nodules are rather hard to distinguish from cirrhotic nodules and precious guidelines recommend a close follow up by repeating US at 3 or 4 months for those patients (35,46). Moreover, a contrast-enhanced CT, MRI or US every 3 to 6 months was advised (25). Earlier researches found that hyperenhancement presented by MRI T2 and diffusion weighted image is of significance to differentiation hyper-vascular HCC nodules smaller than 1cm in diameter (49). Thus, Korean guideline set rigid criteria for diagnosis of HCC nodules less than 1 cm.
Liver nodules with size more than 1 cm ought to receive by dynamic contrast enhanced CT/MRI or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) MRI.
As with non-size-based pathway, patients will ought to undertake dynamic imaging techniques regardless of tumor size. The diagnosis of HCC would be supported by the presentation of an arterial hyperenhancement followed by ‘‘washout’’ in enhanced CT/MRI. Unlike other diagrams, the JSH guideline takes Gd-EOB-DTPA MRI as the first-line surveillance and diagnostic tool for HCC (23). HCC nodules without normal hepatocytes are hypo-intensified, which would assist the differentiation of tumors from non-tumorous nodules (50,51).
In the Japanese Clinical practice guidelines for HCC (J-guideline), the recommendation relied on tumor size (19). When the tumor size was larger than 1 cm, additional tests ought to be scheduled. They included Gd-EOB-DTPA MRI, superparamagnetic iron-oxide (SPIO) MRI, contrast enhanced US, CT angiography and biopsy. However, the selection of further approach only depended on the purpose of the surgery and no specific recommendation was provided in JSH guideline. On the other hand, a follow up of every three months ought to be scheduled for those with tumor size less than 1 cm and increased tumor markers.
When the arterial phase of CT/MRI identified a hypo-vascularity mass, the best diagnostic algorithm was, so far, in debate. The Japanese guideline advocated that additional test ought to be performed for patients with tumor size larger than 1.5 cm and 3 months of follow-up for those with tumor size less than 1.5 cm.
Treatment
A principal purpose of guideline is to collect concrete, convincing evidence currently available to help clinicians adopt the state-of-art therapeutic allocation.
The Barcelona Clinic Liver Cancer (BCLC) staging system overall covering tumor stage, liver function, and physical status was commonly adopted for HCC staging and treatment (52,53). Moreover, it is the only staging system that enables prognostication and informs strategies of first line (54). Here, we briefly and graphically summarized the treatment strategies mainly based on the BCLC staging (Figure 3). Generally, curative strategies contain liver resection, liver transplantation, and ablation. Patients allocated to stage 0 or stage A might get a 5-year survival of 40–70% after the curative intent treatments. Hepatectomy forms the first-line therapy for HCC in non-cirrhotic patients or in selected-cirrhotic patients with a single lesion. Liver transplantation is chosen for patients in BCLC stage A within the Milan criteria (single HCC nodule less than 5 cm in size or fewer than three nodules, none larger than 3 cm in diameter) (55). Patients within the Milan criteria featured a 5-year overall survival of 65–78% after liver transplantation (56). In the view of the Indian National Association for Study of the Liver (INASL) guideline, the University of California San Francisco criterion which expanded the Milan criteria had been validated with identical outcomes (57). Totally speaking, major presentations for liver transplantation stayed unchanged.
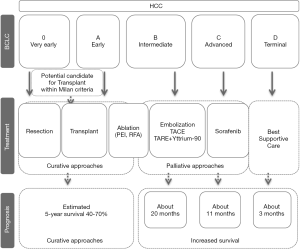
Percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA) are the most commonly utilized ablative arms, which have been taken as therapy of choice for patients belonging to BCLC stage 0–A. Recently, initial treatment by RFA or PEI has returned a comparable result with hepatectomy for BCLC stage 0 patients with tumor size less than 2 cm (58,59).
Transarterial chemoembolization (TACE) serves as the principal therapeutic method for BCLC stage B HCC (60). The recommendations of TACE kept almost the same with that in previous versions. Recent studies have shown transarterial radioembolization could do a better job than TACE to downstage tumor, and its joint use with yttrium-90 microspheres could produce an inspiring prognosis (17,18,20,21,24,61,62).
Sorafenib is indicated for BCLC stage C or BCLC stage B HCC progressed after TACE. RCTs have already demonstrated that sorafenib might act as the treatment of choice for HCC patients with well-preserved liver function who are not suitable for potentially more effective arms (63,64). Concerning update of sorafenib is about certain safety data and the efficacy in survival prolongation (65-67).
Terimnal stage (BCLC stage D) patients ought to be given the best supportive care.
Discussion
In the present study, through a broad search strategy covering major medical publication repositories, 11 main HCC guidelines in Asia were identified. All of the guidelines included were published or finally updated (Most of them have several updated versions.) in the last 8 years and could represent the most recent recommendations in the clinical management of HCC in Asia.
On AGREE II evaluation, overall quality was considered to be moderate among the 11 included guidelines with the majority being suitable for recommendation in practice. Most of the appraisal results stayed in agreement with the current literature (68). The different scores for each domain implied that guideline drafters comparatively placed their focus on different areas. Overall, Korean Guideline obtained the highest score, followed by the J-guideline. Upon closer examination, we found that these guidelines were in compliance with strict methodological rigor (such as the rule of evidence-based recommendation) and updated for several times. This illustrated the drafters’ commitment on continued improvement of their recommendations along with bettering the overall quality of guidelines for clinical practice. Furthermore, it is broadly accepted that evidence based guidelines are superior to non-evidence-based ones in terms of helping clinicians to effectively choose suitable treatment allocations. By contrast, the lowest overall score guideline was the Chinese consensus by Section of Hepatic Surgery, Branch of Surgery, Chinese Medical Association, which was primarily due to a lack of statement of methods and other vital components throughout. However, it was merely expert consensus on selection of surgical treatments for HCC.
By calculating the mean score of each domain, the greatest score of all six domains was found in clarity of presentation, followed by scope and purpose. The former domain is concerned with the language, structure and format of the guideline; and the latter deals with the purpose, clinical issues and target population. This might be explained by the following reasons: (I) most of the guidelines were published in reputable academic journals or books that likely require strict language editing; (II) most of the guidelines had been updated (or modified) several times by a group of multi-disciplinary experts. As to this, commensurate findings were revealed by earlier researches (69-71). Reversely, the applicability domain had the lowest average score, which kept in line with previous researches (69,72). The applicability domain was defined by AGREE II as the facilitators, barriers and resource implications that are associated with guideline use (12). This domain was basic for clinicians, a good score of which indicated the rigor and feasibility of a guideline. Guidelines drafters and users should lay enough priority in this domain. Hence, we propose that it is essential to strengthen the implementation of pilot studies, barrier analyses and clinician feedback aspects when constituting the guidelines (73).
Although analysis was primarily focused on the Asian guidelines, we could not ignore that eastern and western perspectives on clinical practice guidelines for HCC have a lot of commonalities but may also differ in some sections, as described in the present article. This might be due to the regional differences in epidemiology, risk factor, or healthcare policies. Of note, massive new findings about HCC were produced by recent researches, but changes are minor by and large; and they are not easy to take notice of among guidelines. Additionally, there still remain variances in Asian guideline structures and certain contents (particularly in recommendations of surveillance and treatment allocation). For example, the recommendation of AFP use in screening and surveillance was still in dispute. Significances of certain novel biomarkers also require further validation. Hence, better biomarkers are urgently needed for the screening and surveillance of HCC. Similarly, differences in epidemiology which might change with time and region still existed. For example, HBV’s causal role to HCC is weakening; meanwhile the importance of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis as risk factors for HCC are rising (74).
To our best knowledge, there is only one similar article concerning this topic (75). However, it mainly focused on the evaluation of the methodological quality of guidelines concerning HCC resection; additionally, only English guidelines were included for final analysis. Comparatively speaking, there were two main strengths in the present study: (I) this work serves as a study evaluating and comparing current guidelines for the clinical management of HCC in Asia; (II) through a broad search strategy covering major medical publication repositories, main guidelines from different areas in Asia has been incorporated. In spite of this, limitations of the present study had to be taken into consideration: (I) supplementary materials or background information on certain guidelines had been missed and thus could not be reviewed thoroughly. Thus, it is likely that guideline quality in some instances had been underestimated; (II) we did not re-examine the evidence base of the guidelines.
Conclusions
To summarize, the present review might help focus and target advocacy to promote clinical management of HCC in Asian countries. However, further research and more straightforward guidelines are essential to improve the prognosis of HCC in the future.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (NSFC) from China (NO. 81502376), Beijing Natural Science Foundation (NO. 7172201) and Beijing New Star of Science and Technology Foundation (NO. 2017B503).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer 2012;1:226-37. [Crossref] [PubMed]
- Shin HR, Masuyer E, Ferlay J, et al. Cancer in Asia - Incidence rates based on data in cancer incidence in five continents IX (1998-2002). Asian Pac J Cancer Prev 2010;11:11-6. [PubMed]
- Kudo M, Han KH, Kokudo N, et al. Liver cancer working group report. Jpn J Clin Oncol 2010;40:i19-27. [Crossref] [PubMed]
- Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 2006;23:1535-47. [Crossref] [PubMed]
- Nathan H, Hyder O, Mayo SC, et al. Surgical therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-medicare analysis. Ann Surg 2013;258:1022-7. [Crossref] [PubMed]
- Pavlidis N, Hansen H, Stahel R. ESMO clinical recommendations: a practical guide for medical oncologists. Ann Oncol 2007;18:1759-63. [Crossref] [PubMed]
- Utsunomiya T, Shimada M, Kudo M, et al. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg 2015;261:513-20. [Crossref] [PubMed]
- Song P, Tobe RG, Inagaki Y, et al. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int 2012;32:1053-63. [Crossref] [PubMed]
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42. [Crossref] [PubMed]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420-8. [Crossref] [PubMed]
- Chow PK, Choo SP, Ng DC, et al. National Cancer Centre Singapore consensus guidelines for hepatocellular carcinoma. Liver Cancer 2016;5:97-106. [Crossref] [PubMed]
- Section of Hepatic Surgery BoS, Chinese Medical Association. Expert consensus on selection of surgical treatments for hepatocellular carcinoma (2016 3rd edition). Chin J Digest Surg 2016;16:113-5.
- . Ministry of Health P.R.C. The guideline of primary liver cancer. Chin Clin Oncol 2011;16:929-46.
- Poon RT, Cheung TT, Kwok PC, et al. Hong Kong consensus recommendations on the management of hepatocellular carcinoma. Liver Cancer 2015;4:51-69. [Crossref] [PubMed]
- Korean Liver Cancer Study G. National Cancer Center K. 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol 2015;16:465-522. [Crossref] [PubMed]
- Group formed to establish “Guidelines for evidence-based clinical practice for the treatment of liver cancer”. Clinical practice guidelines for hepatocellular carcinoma. 2013 ed. Tokyo, Japan: Kanehara Press; 2013.
- Kumar A, Acharya SK, Singh SP, et al. The Indian National Association for Study of the Liver (INASL) Consensus on Prevention, Diagnosis and Management of Hepatocellular Carcinoma in India: the puri recommendations. J Clin Exp Hepatol 2014;4:S3-S26. [Crossref] [PubMed]
- Abdo AA, Hassanain M, AlJumah A, et al. Saudi guidelines for the diagnosis and management of hepatocellular carcinoma: technical review and practice guidelines. Ann Saudi Med 2012;32:174-99. [Crossref] [PubMed]
- Poon D, Anderson BO, Chen LT, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009;10:1111-8. [Crossref] [PubMed]
- Kudo M, Matsui O, Izumi N, et al. JSH Consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 2014;3:458-68. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Benson AB 3rd, D’Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:563-73. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. [Crossref] [PubMed]
- Ioannou GN, Splan MF, Weiss NS, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2007;5:938-45, 45 e1-4.
- Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis 2010;30:3-16. [Crossref] [PubMed]
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-22. [Crossref] [PubMed]
- Chang PE, Ong WC, Lui HF, et al. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients? A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J Gastroenterol 2008;43:881-8. [Crossref] [PubMed]
- Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 2010;138:493-502. [Crossref] [PubMed]
- McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000;32:842-6. [Crossref] [PubMed]
- Villanueva A, Minguez B, Forner A, et al. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med 2010;61:317-28. [Crossref] [PubMed]
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Bruix J, Sherman M. Practice Guidelines Committee. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [Crossref] [PubMed]
- Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993;328:1797-801. [Crossref] [PubMed]
- Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110-8. [Crossref] [PubMed]
- Huang J, Zeng Y. Current clinical uses of the biomarkers for hepatocellular carcinoma. Drug Discov Ther 2014;8:98-9. [Crossref] [PubMed]
- Song P, Feng X, Inagaki Y, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends 2014;8:266-73. [Crossref] [PubMed]
- . Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res 2010;40:2-144. [PubMed]
- Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015;45. [PubMed]
- Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291-7. [Crossref] [PubMed]
- Bennett GL, Krinsky GA, Abitbol RJ, et al. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol 2002;179:75-80. [Crossref] [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]
- European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii41-8. [Crossref] [PubMed]
- Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57:1592-6. [Crossref] [PubMed]
- Kim JE, Kim SH, Lee SJ, et al. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol 2011;196:W758-65 [Crossref] [PubMed]
- Kudo M. Will Gd-EOB-MRI change the diagnostic algorithm in hepatocellular carcinoma? Oncology 2010;78:87-93. [Crossref] [PubMed]
- Lee JM, Yoon JH, Kim KW. Diagnosis of hepatocellular carcinoma: newer radiological tools. Semin Oncol 2012;39:399-409. [Crossref] [PubMed]
- Richani M, Kolly P, Knoepfli M, et al. Treatment allocation in hepatocellular carcinoma: Assessment of the BCLC algorithm. Ann Hepatol 2016;15:82-90. [Crossref] [PubMed]
- Song P. Standardizing management of hepatocellular carcinoma in China: devising evidence-based clinical practice guidelines. Biosci Trends 2013;7:250-2. [PubMed]
- Sarma S, Sharma B, Chawla YK, et al. Comparison of 7 staging systems in north Indian cohort of hepatocellular carcinoma. Trop Gastroenterol 2010;31:271-8. [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17:S44-57. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587-96. [Crossref] [PubMed]
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82-9. [Crossref] [PubMed]
- Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology 2012;262:1022-33. [Crossref] [PubMed]
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-9. [Crossref] [PubMed]
- Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Zhang T, Ding X, Wei D, et al. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs 2010;21:326-32. [Crossref] [PubMed]
- Terashima T, Yamashita T, Takata N, et al. Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol Res 2016;46:650-6. [Crossref] [PubMed]
- Tomuleasa C, Cristea V, Irimie A. Sorafenib for advanced-stage hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2012;24:346-7. [Crossref] [PubMed]
- Holvoet T, Raevens S, Vandewynckel YP, et al. Systematic review of guidelines for management of intermediate hepatocellular carcinoma using the Appraisal of Guidelines Research and Evaluation II instrument. Dig Liver Dis 2015;47:877-83. [Crossref] [PubMed]
- Huang M, Zhou X. Appraisal of guidelines for androgenetic alopecia using the Appraisal of Guidelines for Research and Evaluation II instrument. J Eval Clin Pract 2015;21:1089-94. [Crossref] [PubMed]
- Gorman SK, Chung MH, Slavik RS, et al. A critical appraisal of the quality of critical care pharmacotherapy clinical practice guidelines and their strength of recommendations. Intensive Care Med 2010;36:1636-43. [Crossref] [PubMed]
- Farghali AA, Al-Khawaja R, Madi L, et al. Rigorous method to assess quality and generalizability of clinical practice guidelines. Can J Hosp Pharm 2014;67:397-8. [Crossref] [PubMed]
- Nasr ZG, Abu Yousef S, Jibril F, et al. Critical appraisal of clinical practice guidelines for adult cancer patients with febrile neutropenia. Int J Pharm Pract 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Sabharwal S, Patel NK, Gauher S, et al. High methodologic quality but poor applicability: assessment of the AAOS guidelines using the AGREE II instrument. Clin Orthop Relat Res 2014;472:1982-8. [Crossref] [PubMed]
- Goh GB, Chang PE, Tan CK. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract Res Clin Gastroenterol 2015;29:919-28. [Crossref] [PubMed]
- Gavriilidis P, Roberts KJ, Askari A, et al. Evaluation of the current guidelines for resection of hepatocellular carcinoma using the Appraisal of Guidelines for Research and Evaluation II instrument. J Hepatol 2017;67:991-8. [Crossref] [PubMed]