
Telomerase reverse transcriptase promoter region mutations and the clinical characteristics of pulmonary neuroendocrine tumors
Introduction
Pulmonary neuroendocrine tumors (NETs) represent approximately 20–25% of all primary lung tumors (1,2). They represent a broad spectrum of morphological types that share specific morphological, ultrastructural, immunohistochemical, and molecular characteristics. NETs have been classified into four categories: low grade typical carcinoid (TC), intermediate grade atypical carcinoid (AC), high grade large cell neuroendocrine carcinoma (LCNEC), and small cell lung carcinoma (SCLC) (3). It is occasionally difficult to distinguish between the different types of pulmonary NETs since the clinical and pathological features, and molecular characteristics of these tumors are not fully understood.
Telomerase reverse transcriptase (TERT) is the catalytic subunit of telomerase, which can effectively maintain the structural integrity of telomeres. Human TERT gene is located on chromosome 5p. TERT promoter mutations mainly occur in the core promoter region 124 (C228T) and 146 (C250T), and these mutations can increase TERT mRNA, protein and enzyme activities, thereby increasing telomere length. Human TERT siRNA can effectively suppress telomerase and lead to apoptosis in A549 lung adenocarcinoma cells (4). Nishio et al. suggested that alterations in telomere length, telomerase activity, and the expression of hTERT mRNA may play roles in the pathogenesis of pulmonary NETs, and can be a useful tool for the differential diagnosis between TCs and LCNECs (5). There is no report about TERT promoter mutations in pulmonary NETs in China. In order to clarify the status of TERT promoter region mutation and the prognosis of pulmonary NET, TERT promoter mutation was analyzed in this study using ARMs-qPCR and Sanger sequencing in resected pulmonary NETs at the Zhejiang Cancer Hospital. The clinical characteristics and prognosis were also analyzed.
Methods
Patients
A total of 41 pulmonary NET specimens obtained from resected tumors were retrospectively collected from the Zhejiang Cancer Hospital in China between 2008 and 2016. The pathological diagnosis was based on the standard criteria defined by the World Health Organization (WHO) (6). The classification of stages was defined by the eighth edition of the TNM classification for lung cancer (7). Clinical characteristics such as gender, age, pathological type, stage, smoking history, and adjuvant chemotherapy cycles received by each patient are listed in Table 1. This study was approved by the Medical Ethical Committee of Zhejiang Cancer Hospital.
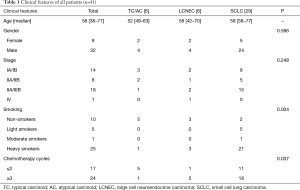
Full table
None of the TC patients received adjuvant chemotherapy, while one AC patient with stage IIIA received adjuvant chemotherapy (four cycles of etoposide and cisplatin) and thoracic radiotherapy. ALL LCNEC cases received >3 cycles of adjuvant chemotherapy. Among the 29 cases of SCLC, 18 cases received >3 cycles of adjuvant chemotherapy (Table 1). Nine patients with stage IIIA and one patient with stage IIIB received adjuvant thoracic radiotherapy. Only four patients received prophylactic cranial irradiation (PCI) including three stage IIIA cases and one stage IIIB case.
Analysis of TERT mutations
Mutational analyses of TERT (C228T and C250T) were performed on genomic DNA extracted from paraffin-embedded tumor tissues using ARMs-qPCR (Bio-Rad qPCR, CFX96) and sanger sequencing. The protocol of qPCR was as follows: The reaction mix was prepared with enzyme, primer, probe, MgCl2 (25 mM), dUTP (2 mM), Uracil DNA glycosylase (UNG) and heat-labile. Then, the mix (18 mL/well), sample DNA (10 ng/µL, 2 µL/well) and reference standard DNA (2 µL/well), respectively, were added to a 96-well-plate, which was sealed and centrifuged for 30 s at 3,700 rpm. Sequencing was performed with BIO-RAD CFX96 PCR detection system for one hour, and the 6-Carboxyfluorescein (FAM) and rhodamine-X (ROX) signals were collected. The results were statistically analyzed with matched software. The protocol for Sanger sequencing was as follows: A total of 19 µL of reaction mix including PCR reaction enzyme, primer and ddH2O was combined with 1 µL of sample DNA (10 ng/µL). PCR was performed with 2,720 Thermal Cycler PCR detection system for 2 hours and 10 minutes, then sequencing was performed with ABI3730. Finally, the results were statistically analyzed with Mutation Surveyor V4.0.8 (demo).
Follow-up
The follow-up deadline was May 05, 2017. The survival time was calculated from the date of pathological diagnosis.
Statistical analyses
The data were analyzed using the statistical package for the social sciences (SPSS) software version 15.0. Overall data were screened using the Chi-square test. The survival curves were calculated by the Kaplan-Meier method, and compared using the log-rank test, with P<0.05 indicating statistical significance.
Results
Population characteristics
Most of the patients were male (76.2%). The median age of TC/AC, LCNEC and SCLC patients was >50 years. Most of the patients were heavy smokers in the SCLC group (72.4%), but non-smokers in the TC/AC group (83.3%).
Status of TERT mutation
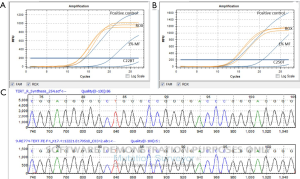
No TERT promoter region 124 (C228T) and 146 (C250T) mutation was found among the 41 cases of pulmonary NET (Figure 1).
Survival analyses
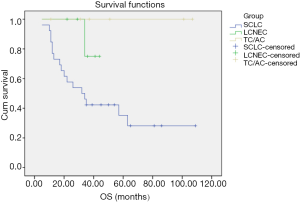
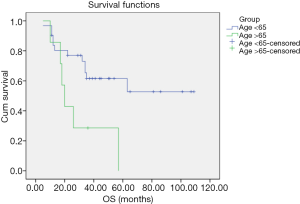
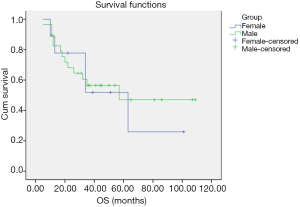
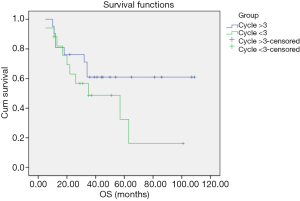
No TC/AC patient was lost to follow-up. All TC/AC patients were alive with no relapse, and the follow-up duration was 11–107 months. No LCNEC patient was lost to follow-up, and the follow-up duration was 22–44 months. One patient suffered from cervical vertebra metastasis and died 34 months after pathological diagnosis. One LCNEC patient with brain metastasis underwent surgery and is currently alive (≥29 months). One patient suffered from thoracic vertebra metastasis 10 months after the pathological diagnosis and is currently alive (≥34 months). The other three LCNEC patients are currently alive with no relapse. Three patients were lost to follow-up, 17 patients died and nine patients were alive among the 29 SCLC patients. The follow-up duration was 5–109 months. Overall survival (OS) of the different histological subtypes of pulmonary NET is shown in Figure 2. OS of the SCLC patients ≤65 and >65 years old is shown in Figure 3. OS of the female and male SCLC patients is shown in Figure 4. OS of the SCLC patients who received ≤2 cycles of adjuvant chemotherapy and those who received ≥3 cycles of adjuvant chemotherapy is shown in Figure 5.
Discussion
TERT is the catalytic component of telomerase, and the rate-limiting determinant of telomerase activity (8). Telomerase activation is essential for carcinogenesis (9). TERT promoter mutations frequently occur in older patients, males, with high tumor grade and stage. It is significantly associated with distant metastasis and serves as an adverse prognostic factor (10). TERT promoter mutations were seen in NSCLC patients from China, the United States and European countries (11,12). The frequencies of TERT promoter mutations among squamous cell carcinomas (SCCs) at different sites are as follows: ~70% in skin SCC and urothelial carcinoma with squamous differentiation, 16.67% in head and neck SCC, and none in lung and cervical SCC (13). Data from the United States showed that the frequency of TERT promoter mutation in small cell carcinoma is low, except for those originating from the bladder (14). Next generation sequencing (NGS) was applied to 148 pulmonary NET from Italy, comprising of the four WHO classification categories. Multivariate survival analysis revealed RB1 mutation (P=0.0005) and TERT copy gain (P=0.016) as independent predictors of poorer prognosis. No TERT mutation was found in these 148 pulmonary NET patients (15). The incidence of EGFR mutation in NSCLC is higher in China than in the United States and European countries (16,17). TERT polymorphisms of NSCLC are higher in Asians than in Europeans (18,19). In China, TERT polymorphisms are more strongly associated with the risk of NSCLC with EGFR mutation than those without EGFR mutation (19). Whether there is a difference in TERT promoter region 124 (C228T) and 146 (C250T) mutations between Chinese as compared to Americans and Europeans remains unknown. Our study showed no TERT promoter region 124 (C228T) and 146 (C250T) mutations in 41 cases of pulmonary NET. The consistency of TERT promoter region 124 (C228T) and 146 (C250T) mutation of pulmonary NET from China and Italy is different from the inconsistency of EGFR mutation in Chinese and Caucasians (14-17). These data suggested that TERT promoter region 124 (C228T) and 146 (C250T) mutations may not be a useful biomarker to distinguish between the different subtypes of pulmonary NET.
The OS curve of the subtypes of pulmonary NET showed that the prognosis of TC/AC was better than SCLC and LCNEC, which may be due to the different differentiations and malignancies of these subtypes. The prognosis of these subtypes is consistent with other reports (20-23). Yang et al. reported the role of adjuvant therapy in a population-based cohort of patients with early stage SCLC. The results showed that the adjuvant chemotherapy was associated with significantly improved survival as compared to surgery alone (23). In our cohort, SCLC patients who received >3 cycles of adjuvant chemotherapy had a longer OS as compared to those with ≤2 cycles of chemotherapy. Kim et al. showed that OS was significantly improved in younger than in older patients with local disease SCLC (LD-SCLC) (24), which was consistent with our findings.
Pulmonary NETs tend to occur in the fourth to sixth decade of life (25,26). The median ages of TC/AC, LCNEC, and SCLC were 52, 58 and 58 years in our cohort. Another report from China showed that 215 (72.1%) patients were <65 years old at the time of diagnosis (27). The majority of ACs/TCs occurred in never or light smokers (26). In our cohort, most of the AC/TC patients were non-smokers. SCLC is a smoking-related disease and most of the patients in our study were heavy smokers. It is consistent with another report from China in which 208 patients (69.8%) had a smoking history (27). The proportion of women with SCLC increased from 28% in 1973 to 50% in 2002 as per the data from the surveillance, epidemiologic, and end results (SEER) database (28). Our study suggested that most of the SCLC patients were male and another report from China also showed that 235 (78.9%) of the 298 SCLC patients were male (27). Male to female ratios of pulmonary TC/AC were 0.8 as recorded in SEER (29), while most of the TC/AC patients were male in our cohort. Another report showed that LCNEC frequently occurred in males and smokers (22,30). In our cohort, 50% of the LCNEC patients were male and heavy smokers.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (No. 81303213), Zhejiang province public welfare and technology application project of China (2016C33118), Zhejiang province traditional medical science project of China (No. 2015ZA037), and the 1022 Talent Training Program of Zhejiang Cancer Hospital.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.12.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethical Committee of Zhejiang Cancer Hospital (IRB-2016-86). Most of the patients in this retrospective study signed the written informed consent before surgery to preserve their specimens in the Biological Sample Bank of Zhejiang Cancer Hospital to be used in research. This study is a retrospective study and a number of patients have died, exempt written informed consent was also approved by the Ethics Committee of Zhejiang Cancer Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Langfort R, Rudziński P, Burakowska B. Pulmonary neuroendocrine tumors. The spectrum of histologic subtypes and current concept on diagnosis and treatment. Pneumonol Alergol Pol 2010;78:33-46. [PubMed]
- Xie M, Chen Q, He S, et al. Silencing of the human TERT gene by RNAi inhibits A549 lung adenocarcinoma cell growth in vitro and in vivo. Oncol Rep 2011;26:1019-27. [PubMed]
- Nishio Y, Nakanishi K, Ozeki Y, et al. Telomere length, telomerase activity, and expressions of human telomerase mRNA component (hTERC) and human telomerase reverse transcriptase (hTERT) mRNA in pulmonary neuroendocrine tumors. Jpn J Clin Oncol 2007;37:16-22. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Wu XQ, Huang C, He X, et al. Feedback regulation of telomerase reverse transcriptase: new insight into the evolving field of telomerase in cancer. Cell Signal 2013;25:2462-8. [Crossref] [PubMed]
- Xie H, Liu T, Wang N, et al. TERT promoter mutations and gene amplification: promoting TERT expression in Merkel cell carcinoma. Oncotarget 2014;5:10048-57. [Crossref] [PubMed]
- Yuan P, Cao JL, Abuduwufuer A, et al. Clinical characteristics and prognostic significance of TERT promoter mutations in cancer: a cohort study and a meta-analysis. PLoS One 2016;11:e0146803 [Crossref] [PubMed]
- Ma X, Gong R, Wang R, et al. Recurrent TERT promoter mutations in non-small cell lung cancers. Lung Cancer 2014;86:369-73. [Crossref] [PubMed]
- Li C, Hao L, Li Y, et al. Prognostic value analysis of mutational and clinicopathological factors in non-small cell lung cancer. PLoS One 2014;9:e107276 [Crossref] [PubMed]
- Cheng KA, Kurtis B, Babayeva S, et al. Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites. Ann Diagn Pathol 2015;19:146-8. [Crossref] [PubMed]
- Zheng X, Zhuge J, Bezerra SM, et al. High frequency of TERT promoter mutation in small cell carcinoma of bladder, but not in small cell carcinoma of other origins. J Hematol Oncol 2014;7:47. [Crossref] [PubMed]
- Simbolo M, Mafficini A, Sikora KO, et al. Lung neuroendocrine tumours: deep sequencing of the four WHO histotypes reveals chromatin remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol 2017;241:488-500. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Wu YL, Zhong WZ, Li LY, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007;2:430-9. [Crossref] [PubMed]
- Landi MT, Chatterjee N, Yu K, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet 2009;85:679-91. [Crossref] [PubMed]
- Wei R, Cao L, Pu H, et al. TERT polymorphism rs2736100-C is associated with EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res 2015;21:5173-80. [Crossref] [PubMed]
- Hilal T. Current understanding and approach to well differentiated lung neuroendocrine tumors: an update on classification and management. Ther Adv Med Oncol 2017;9:189-99. [Crossref] [PubMed]
- Wolin EM. Advances in the diagnosis and management of well-differentiated and intermediate-differentiated neuroendocrine tumors of the lung. Chest 2017;151:1141-6. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Evangelista A, et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: results from the European Society for Thoracic Surgeons Lung Neuroendocrine Tumours Retrospective Database. Eur J Cardiothorac Surg 2017;52:339-45. [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of adjuvant therapy in a population based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Kim HJ, Choi CM, Kim SG. The younger patients have more better prognosis in limited disease small cell lung cancer. Tuberc Respir Dis (Seoul) 2016;79:274-81. [Crossref] [PubMed]
- Faggiano A, Ferolla P, Grimaldi F, et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: the NET management study. J Endocrinol Invest 2012;35:817-23. [PubMed]
- Hassan MM, Phan A, Li D, et al. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 2008;123:867-73. [Crossref] [PubMed]
- Shi Y, Xing P, Fan Y, et al. Current small cell lung cancer treatment in China. Thorac Cancer 2015;6:233-8. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [Crossref] [PubMed]
- Kim KW, Kim HK, Kim J, et al. Outcomes of curative-intent surgery and adjuvant treatment for pulmonary large cell neuroendocrine carcinoma. World J Surg 2017;41:1820-7. [Crossref] [PubMed]


