
Prognostic significance of neutrophil-lymphocyte ratio in multiple myeloma patients
Introduction
Multiple myeloma (MM) is a hematological malignant disease characterized by the proliferation of monoclonal plasma cells. This disease causes numerous clinical complications, including elevated calcium, renal failure, anemia, bone lesions, opportunistic infections and weight loss (1). Although novel therapeutic measures, such as steroids, chemotherapy, thalidomide or lenalidomide and stem cell transplant have been developed to yield improved clinical outcomes, the relapse rate and mortality remain high and the prognosis of MM is also highly heterogeneous (2). Thus, the accurate and rapid prediction of disease prognosis is essential for treatment planning. Prognostic factors, including cytogenetics and International Staging System (ISS), have been proposed, but cytogenetics requires complex detection methods and expensive costs. Although ISS, which is based on albumin and β2 microglobulin, is simpler and less expensive than other methods, the prognosis of Asian patients through ISS is weaker than that of Westerners. Data used to build ISS were obtained before 2002 when few patients accepted novel agents (2). Therefore, simple, accurate and inexpensive tumor markers should be established to predict recurrence and poor outcomes.
Chronic inflammation plays a positive role in tumor progression. A tumor microenvironment exists in tumor-type-specific manners. Inflammatory cells in tumor microenvironments induce the proliferation and survival of cancer cells, promote angiogenesis and metastasis, and depress antitumor immunity (3), and inflammatory factors in clinical practice may be more easily and cost effectively detected. Neutrophils and lymphocytes are regarded as cardinal cells closely correlated with local inflammation and immune responses (4) and can be easily collected from complete blood. Neutrophil-to-lymphocyte ratio (NLR) indicates the balance between pro-tumor and anti-tumor status and thus a useful index to predict the prognosis of patients with malignant tumors.
NLR associated with poor prognosis in many tumor types, including lung cancer, laryngeal carcinoma, bladder cancer, colorectal cancer, and rectal cancer, has been comprehensively examined (5-15). However, we have yet to determine the role of NLR in predicting the prognosis of MM when bortezomib was developed. In this study, patients with MM treated with bortezomib-thalidomide-dexamethasone (VTD) in our hospital were retrospectively analyzed to investigate the association between NLR and prognosis in Chinese MM patients and to determine the optimum value for the screening of patients with poor prognosis.
Methods
Patients and data collection
A total of 157 patients newly diagnosed with MM and subjected to bortezomib-thalidomide-dexamethasone chemotherapy for at least three cycles were followed up at the Nanjing Drum Tower Hospital between 2008 and 2016. Two patients were excluded because pretreatment NLR and pretreatment bone marrow biopsy were unavailable. Eleven patients who undergo autotransplantation, and eight patients who changed to cyclophosphamide-thalidomide-dexamethasone chemotherapy after one cycle of bortezomib-thalidomide-dexamethasone chemotherapy, were also excluded. The data of the 136 remaining patients were complete blood count (CBC), serum calcium, inorganic phosphorus, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), serum β2 microglobulin and the results of FISH analyses. The data obtained from CBC were used to calculate NLR. All of the patients provided informed consent for data analysis in compliance with the Declaration of Helsinki, and the existence of other treatment options was explained in accordance with the Ethics Committee of Nanjing University.
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis to the day of death from any cause or the last day the patient was known to be alive. Progression-free survival (PFS) was determined from the date of inducing chemotherapy to death or disease progression. The prognostic value of NLR was analyzed by establishing a receiver operating characteristic (ROC) curve to select the cutoff. Pearson chi-squared or Fisher’s exact test was used to assess the association between NLR and clinical characteristics. Mann-Whitney text was used to compare the values of NLR between patients with and without genetic alterations. Kaplan-Meier method was employed to estimate survival probabilities, and log-rank test was carried out to compare survival differences. Prognostic factors were subjected to multivariate analysis by using Cox proportional hazards method with the following variables: age, gender, hemoglobin (HB), red blood cell distribution width (RDW), serum calcium, inorganic phosphorus, ALP, LDH, BUN, serum β2 microglobulin, and NLR. All data were analyzed using SPSS 13.0 (IBM Corp, Armonk, NY, USA). P<0.05 was considered significant, and two-sided tests were conducted in all of the calculations.
Results
Clinical characteristics
A total of 136 patients were enrolled in this study. The median age of the study patients was 61 years (range, 40 to 80 years). A total of 73 males (53.7%) were examined. According to ISS, 106 patients were in stage II and accounted for the largest proportion (77.9%), 10.3% of the patients were in stage I, and 11.8% of the patients were in stage III. The estimated median OS was 27 months, and the median PFS was 17 months. Eight patients died during follow up. The median absolute neutrophil count was 2.6×109, with a ranging of 0.7×109 to 8×109. The median absolute lymphocyte count was 1.5×109, with a range from 0.4×109 to 6.5×109. HB ranged from 39 to 158 g/L, and its median was 92 g/L. Other clinical characteristics are summarized in Table 1.
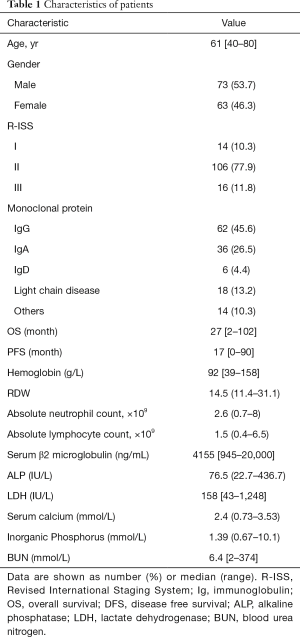
Full table
Prognostic variables of NLR
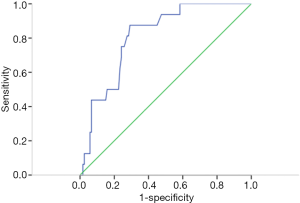
The ROC curve confirmed that 2 was the optimal cutoff point to discriminate between the survival and death of patients in our study. In Figure 1, the area under the curve of NLR was 80.9% (P=0.000).
Patient characteristics according to NLR
The correlations between NLR and clinical characteristics are shown in Table 2. The patients were divided into different groups based on the cutoff value of NLR. Eight-seven patients presented values of NLR <2, and forty-nine patients had NLR ≥2. No significant differences were found between the two groups in age, gender, type of monoclonal protein, levels of serum β2 microglobulin, RDW, ALP, and inorganic phosphorus. By contrast, patients with high NLR were significant difference from patients with low NLR in the ISS (P=0.000), HB level (P=0.029), LDH (P=0.008), calcium (P=0.001), and BUN (P=0.017), which were associated with poor prognosis, such as anemia, skeletal destruction, and renal failure. The ISS of the patients in the high-NLR group was more advanced than those in the low-NLR group. The percent of patients with low HB in the high-NLR group exhibited a larger percent than the other group (28.6% vs. 8.0%). The same phenomenon was identified in the content of BUN (51.7% vs. 35.6%).
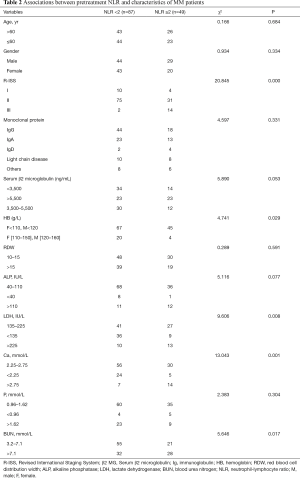
Full table
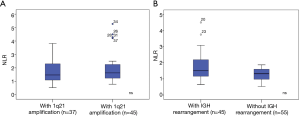
The results of FISH analyses of 82 MM patients were collected (Figure 2). However, no significant differences were observed in the value of NLR between patients with and without 1q21 amplification. And the same phenomenon was found between patients with and without IGH rearrangement.
Survival analysis
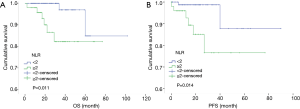
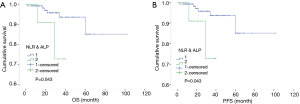
The Kaplan-Meier survival analysis demonstrated that patients in the high-NLR group had poorer OS and PFS than those in the low-NLR group (χ2=6.503, 6.087, P=0.011, 0.014) (Figure 3). The 5-year OS and PFS in the high-NLR and low-NLR groups were 82.20% vs. 96.67% and 76.58% vs. 87.76%, respectively. The multivariate survival analysis is shown in Table 3. In the Cox survival analysis, NLR at diagnosis was an independent prognostic factor for MM patients [95% confidence interval (CI): 0.012 to 0.783, 0.005 to 0.596, P=0.028, 0.018]. ALP also remained as a prognostic factor (95% CI: 0.023 to 0.942, 0.025 to 0.965, P=0.043 and 0.046). Other potential prognostic factors, including HB, RDW, and serum β2 microglobulin, were not significantly correlated with OS and PFS (P>0.05). NLR and ALP were two prognostic factors for MM patients. Therefore, we combined NLR and ALP to explore whether such a combination could improve the predicting effect of prognosis on MM. We divided patients into two groups. One group comprised patients with high NLR and deviant ALP (the ALP level did not belong to the range from 40 to 110 IU/L), and the other group consisted of the remaining patients. The numbers of patients in the two groups were 13 and 123. The first group had shorter OS time (35 vs. 93 months) and PFS time (28 vs. 82 months), and the difference was statistically significant (χ2=4.097, 4.028, P=0.043, 0.043) (Figure 4).
Full table
Discussion
The prediction of prognosis has been playing an important role in treatment planning for MM patients. Inflammation is closely related to tumor progression (3), and neutrophils and lymphocytes are the main cells to participate in inflammation and immune responses (4). Accordingly, we studied the data of patients treated in our hospital to determine the association between NLR and prediction of prognosis in MM. This study showed that the NLR value in MM patients was a risk factor for recurrence-free and cancer specific survivals. Patients with NLR ≥2 had short durations of OS and PFS, advanced stage, high risk of anemia, skeletal destruction, and renal insufficiency. Combination of NLR and ALP could be a prognostic factor for patients with MM.
Many inflammatory indexes are related to the prognosis in MM, such as ALP (16-18). Numerous investigators have paid close attention to the prognostic value of NLR in malignancies in the last few years (5-15). A meta-analysis, including 15 studies about the prognostic role of NLR in breast cancer, concluded that high NLR was associated with adverse OS and PFS (19). Wei conducted a meta-analysis about the prognostic role of NLR in urinary cancers and obtained the same conclusion (20). Although several studies about solid tumors exist, investigators have begun focused on hematological malignancies. Keam reported that pre-NLR ≥3 was an independent predictor for the poor prognosis of patients with diffuse large B-cell lymphoma (21). Another study has yielded the same conclusion (22).
High NLRs in patients with MM are associated with negative prognosis (23-26). However, the cutoff value of NLR was different in four studies. Li (23) selected NLR =2 as the cutoff value; Shi (24) set NLR =4; Kim (25) obtained NLR =2.25; Wongrakpanich (26) set it at NHL=2.78. The reasons for the differences among three studies might be related to their different areas and the accuracy of detecting instruments. We analyzed the data of patients in our hospital to determine the most befitting cutoff values. We obtained a cutoff value of 2, which was similar to that identified by Li. All of these studies were about patients in East Asia. Hence, patients with NLR <2 might have a better prognosis. We combined NLR and ALP to improve the efficiency of NLR in predicting MM prognosis and found that patients with high NLR and deviant ALP yielded significantly short OS and PFS. This conclusion was not presented in the other studies.
Although cytogenetics has been reported to show a prognostic value in MM (27,28), it is not a routine pretreatment assessment in grass root hospitals, especially those in economically less-developed areas. On the contrary, hematological test is conducted routinely as an inexpensive and convenient laboratory parameter before the treatment of cancer patients.
The specific mechanism involved in the interaction between increased NLR and poor prognosis of cancer is incompletely understood. Some possible explanations can be used to interpret this result. First, lymphocytes play an important role in the antitumor immunological reaction by preventing the proliferation and metastasis of malignant cells (29). Systemic inflammation response from malignant cells can cause immune suppression by which tumor cells can escape from host immune reaction (30). Second, many types of tumor tissues are infiltrated by neutrophils. Tumor-associated neutrophils are related to progress in cancer for they are the primary source of circulating vascular endothelial growth factor (VEGF), which can accelerate tumor-related angiogenesis (31,32). Neutrophils directly help tumor cells survive by inducing proliferation (33). Therefore, increased NLR, caused by an increase in the number of neutrophils and a decrease in lymphocyte count, indicates that the balance between pro-tumor and anti-tumor status has been disrupted and skewed to a pro-tumor inflammatory condition, which leads to tumor progress and poor prognosis.
However, this study is limited by some factors. For instance, the sample size used in this study was relatively small. Therefore, outcomes, such as NLR cutoffs, should be confirmed with a large, multicenter study. Additionally, the stage of the disease cannot be precisely judged because of the retrospective study design. Further analysis also should be performed on the mechanism of increased NLR, which contributes to poor prognosis.
In conclusion, this study demonstrated that NLR ≥2 upon diagnosis was associated with poor prognosis of MM patients treated with bortezomib-thalidomide-dexamethasone chemotherapy, and this parameter might be an important marker for the outcome of therapy in patients with MM in the Chinese population.
Acknowledgments
We would like to thank all the volunteers who took part in this study.
Funding: This work was supported by the National Natural Science Foundation of China [81570174], the Six Talent Peaks Project of Jiangsu Province (2015-WSN-075),the Jiangsu Provincial Medical Innovation Team (CXTDA2017046), the Jiangsu Provincial Medical Youth Talent (QNRC2016039),and the Technique Development Foundation of Nan Jing [(Outstanding Youth Foundation, JQX15004),YKK16099].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethical and Protocol Review Committee of Nanjing University. All procedures performed in studies involving human participants were in accordance with the ethical standards of Nanjing University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Plasma Cell Neoplasms (Including Multiple Myeloma) Patient Version. NCI. Available online: https://www.cancer.gov/types/myeloma
- Iriuchishima H, Saitoh T, Handa H, et al. A new staging system to predict prognosis of patients with multiple myeloma in an era of novel therapeutic agents. Eur J Haematol 2015;94:145-51. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Xue TC, Zhang L, Xie XY, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. PLoS One 2014;9:e96072 [Crossref] [PubMed]
- Takahashi Y, Horio H, Hato T, et al. Neutrophil-Lymphocyte Ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol 2015;22:S1324-31. [Crossref] [PubMed]
- Duzlu M, Karamert R, Tutar H, et al. Neutrophil-lymphocyte ratio findings and larynx carcinoma: a preliminary study in Turkey. Asian Pac J Cancer Prev 2015;16:351-4. [Crossref] [PubMed]
- Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lym phocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350-61. [Crossref] [PubMed]
- Ock CY, Nam AR, Lee J, et al. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer 2017;20:254-62. [Crossref] [PubMed]
- Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil-to -lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol 2016;23:646-54. [Crossref] [PubMed]
- Kosumi K, Baba Y, Ishimoto T, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carnoma patients. Surg Today 2016;46:405-13. [Crossref] [PubMed]
- Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 2015;22:670-6. [Crossref] [PubMed]
- Wei K, Wang M, Zhang W, et al. Neutrophil-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma undergoing TAE combined with Sorafenib. Med Oncol 2014;31:969-76. [Crossref] [PubMed]
- Kaynar M, Yıldırım ME, Badem H, et al. Bladder cancer invasion predictability based on preoperative neutrophil-lymphocyte ratio. Tumour Biol 2014;35:6601-5. [Crossref] [PubMed]
- Tohme S, Sukato D, Chalhoub D, et al. Neutrophil-lymphocyte ratio is a simple and novel biomarker for prediction of survival after radioembolization for metastatic colorectal cancer. Ann Surg Oncol 2015;22:1701-7. [Crossref] [PubMed]
- Chu-Yuan H, Jing P, Yi-Sheng W, et al. The impact of chemotherapy-associated neutrophil/lymphocyte counts on prognosis of adjuvant chemotherapy in colorectal cancer. BMC Cancer 2013;13:177-87. [Crossref] [PubMed]
- Poudel B, Mittal A, Shrestha R, et al. Liver involvement in multiple myeloma: a hospital based retrospective study. Asian Pac J Cancer Prev 2012;13:2153-5. [Crossref] [PubMed]
- Minauchi K, Shima K, Hashiguchi J, et al. Serum ALP elevation early after treatment is a predictor for response in myeloma patients treated with bortezomib. Rinsho Ketsueki 2015;56:1064-8. [PubMed]
- Zangari M, Aujay M, Zhan F, et al. Alkaline phosphatase variation during carfilzomib treatment is associated with best response in multiple myeloma patients. Eur J Haematol 2011;86:484-7. [Crossref] [PubMed]
- Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2-14. [Crossref] [PubMed]
- Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS One 2014;9:e92079 [Crossref] [PubMed]
- Keam B, Ha H, Kim TM, et al. Neutrophil to lymphocyte ratio improves prognostic prediction of International Prognostic Index for patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 2015;56:2032-8. [Crossref] [PubMed]
- Wang J, Zhou M, Xu JY, et al. Prognostic role of pretreatment neutrophil-lymphocyte ratio in patients with diffuse large B-cell lymphoma treated with RCHOP. Medicine (Baltimore) 2016;95:e4893 [Crossref] [PubMed]
- Li Y, Li H, Li W, et al. Pretreatment neutrophil/lymphocyte ratio but not platelet/lymphocyte ratio has a prognosis impact in multiple myeloma. J Clin Lab Anal 2017;31.
- Shi L, Qin X, Wang H, et al. Elevated neutrophil-to lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 2017;8:18792-801. [PubMed]
- Kim DS, Yu ES, Kang KW, et al. Myeloma prognostic index at diagnosis might be a prognostic marker in patients newly diagnosed with multiple myeloma. Korean J Intern Med 2017;32:711-21. [Crossref] [PubMed]
- Wongrakpanich S, George G, Chaiwatcharayut W, et al. The Prognostic Significance of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Patients With Multiple Myeloma. J Clin Lab Anal 2016;30:1208-13. [Crossref] [PubMed]
- Kim M, Ju YS, Lee EJ, et al. Abnormalities in cgromosomes 1q and 13 independently correlate with factors of poor prognosis in multiple myeloma. Ann Lab Med 2016;36:573-82. [Crossref] [PubMed]
- Liu N, Zhou H, Yang G, et al. Retrospective analysis of genetic abnormalities and survival in 131 patients with multiple myeloma. Oncol Lett 2015;9:930-6. [Crossref] [PubMed]
- Zheng RR, Huang M, Jin C, et al. Cervical cancer systemic inflammation score: a novel predictor of prognosis. Oncotarget 2016;7:15230-42. [Crossref] [PubMed]
- Martinet L, Garrido I, Filleron T, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011;71:5678-87. [Crossref] [PubMed]
- Lu LC, Hsu CH. Tumor-associated neutrophils: an emerging player in the immune microenvironment of hepatocellular carcinoma. Transl Cancer Res 2016;5:S296-9. [Crossref]
- Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol 2016;28:187-96. [Crossref] [PubMed]
- Wu J, Chen M, Liang C, et al. Prognostic value of the pretreatment neutrophil- to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Oncotarget 2017;8:13400-12. [PubMed]


