
Enhanced antitumor effect via combination of triptolide with 5-fluorouracil in pancreatic cancer
Introduction
Pancreatic cancer is a type of malignant tumor with poor prognosis. Only about 15% to 20% of the patients were able to accept surgical treatment and the 5-year survival rate is less than 1% (1). In order to improve the therapeutic effect, a variety of chemotherapeutic drugs and molecular targeted drugs were used to treat patients with advanced pancreatic cancer (2). Combined therapy can not only reduce the toxicity of chemotherapy drugs to the body but also enhance the sensitivity of tumor cells to drugs. How to enhance the effect of chemotherapy and prevent tumor recurrence and metastasis becomes an urgent research question. Although gemcitabine is the first line chemotherapy for pancreatic cancer, the treatment effect is not satisfactory due to the drug resistance of pancreatic cancer (3). For patients who fail to gemcitabine chemotherapy, the selective use of second-line chemotherapy such as 5-fluorouracil (5-FU) may also be effective (4).
Triptolide (TPL) is one of the main active ingredients of Tripterygium wilfordii, which has anti infection, immunity, antitumor and other pharmacological effects (5,6). TPL has been reported with a correlation to the inhibition of nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2) expression, heat shock protein (HSP) expression, nuclear transcription factor (NF-kB) activation pathway and the induction the apoptosis of tumor cells (7-9). TPL can not only induce tumor cells apoptosis but also has the synergistic effect to induce apoptosis; the mechanism may be correlated with the activation of NF-kB, promoting the expression of p53, the activation of MAP kinase and PARP, and caspase cleavage is also involved in the induction of cell apoptosis process (10-12). TPL has been reported to significantly improve the 5-FU effect in colorectal cancer cells (13). 5-FU normally acts as the preferred anti-metabolism drugs for digestive system cancer including pancreatic cancer, which mainly inhibits S DNA synthesis and block G1-S phase as its anti-tumor mechanism (14). It was also reported that TPL combined with gemcitabine to enhance antitumor effect on pancreatic cancer cells (15). Based on the above reviewed studies, we use the TPL alone or combined with 5-FU to treat pancreatic cancer cells AsPC-1. This analysis aims to find out the key protein involved in TPL in combination with 5-FU on pancreatic cancer apoptosis which plays an important role in resistance to chemotherapeutic drugs.
Vimentin can be added to the list of intermediate filament proteins, including the nuclear lamins and cytokeratins 18 and 19, which are cleaved by caspases during apoptotic cell death (16). The vimentin proteolysis occurs secondary to caspase pathway activation (16,17). Vimentin proteolysis releases potential pro-apoptotic proteolytic fragments that can markedly enhance apoptosis (16,17). Therefore, vimentin protein was investigated to be involved in the enhanced mechanism.
In this study, we explore the effect of TPL used alone or in combination with 5-FU and attempt to evaluate this new therapeutic approach in controlling pancreatic cancer. The underlying mechanism by which TPL exerts its enhancement effect on pancreatic cancer was also explored. This study aims to find out the key protein involved in TPL in combination with 5-FU on pancreatic cancer apoptosis which plays an important role in resistance to chemotherapeutic drugs.
Methods
Cells and reagents
AsPC-1, a human pancreatic cancer cell line, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained as monolayer cultures in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 µg/mL of streptomycin and 100 U/mL of penicillin. The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
TPL with 99.9% purity was purchased from the Institute of Medical Research (Fuzhou, China), 5-FU was purchased from Changchun Changqing Pharmaceutical Group Company, sulforhodamine B (SRB) from Sigma (Louis, MO, USA), Caspase-3 Assay Kit from Molecular Probes (OR, USA), cleaved caspase-3 and vimentin monoclonal antibodies from Cell Signaling Technology, Inc. (Beverly, MA).
Cell viability assay
Viability of AsPC-1after different treatments was evaluated by SRB assay. Briefly, AsPC-1cells (1×103 cells/well) were plated in triplicates in 96-well plates and 24 hours later and the fresh media was added with different concentrations of TPL or 5-FU alone and TPL plus 5-FU. The control cells were treated with the same concentration of vehicle (0.01% DMSO). After further incubation for 24, 48 and 72 hours respectively, 50 µL of 10% trichloroacetic acid was added to fix cells at 4 °C for 2 hours, stained with 70 µL of 0.3% SRB for 30 min, color developed with 200 µL Tris base (10 mM, pH=10.5), and read at A490.
Flow cytometric analysis of apoptosis
Forty-eight hours after treatment with TPL alone, 5-FU alone or TPL plus 5-FU, cells were harvested and stained with Annexin V for 30 min and then with PI right before the analysis with flow cytometer according to the manufacturer’s instructions. The percentage of cells that were Annexin V positive and PI positive was compared among the different treatment groups. The data were analyzed with the CellQuest software. Each sample was performed in duplication and the experiment was conducted with three repetitions.
Assays for activity of caspase-3
The activity of caspases 3 was measured with fluorescent substrate assay according to the instruction of the manufacturer (Molecular Probes, OR, USA). Briefly, 1×106 cells treated with either vehicle alone or TPL or 5-FU alone or both in combination for 24, 48 and 72 hrs were collected and resuspended in cold lysis buffer. 50 µL of 2× reaction buffer was added to 50 µL of cell lysate and incubated for 2 h at 37 °C with DEVD-R110, a caspase-3 substrate, which released fluorescence after its cleavage by fluorometer.
Western blot for cleaved caspase-3 and vimentin
The cleavage of caspase-3 is a characteristic marker of apoptosis. For detection this, AsPC-1cells in 100 mm dishes at 80% confluence were treated vehicle alone, TPL or 5-FU or in combination for 24–72 hours, and then harvested with 1 mL of lysis buffer (1% Triton X-100, 0.5% Na deoxycholate, 0.5 µg/mL leupetin, 1 mM EDTA, 1 µg/mL pepstatin, and 0.5 mM phenylmethylsulfonyl fluoride). The protein concentration of the lysate was determined by the bicinchoninic acid method (Pierce, Rockford, IL, USA). The protein was loaded onto a 10% SDS-PAGE, electrophoresed, and transferred to a nitrocellulose membrane. The loading and transferring of equal amounts of protein were confirmed by staining the membrane with a Ponceau S solution (Sigma). The membranes were blocked with 5% fat-free milk in PBS (pH=7.4) for 30 min and then incubated overnight with 0.2 µg/mL of the anti-caspase-3 monoclonal antibody, which recognizes the 17 kDa cleavage fragment. After washing, the membranes were incubated with HRP-labeled secondary antibodies for an hour followed with ECL exposure. For re-probing-tubulin, the blots were stripped with a buffer containing 50 mM Tris-HCl (pH=6.8), 2% sodium dodecyl sulfate, and 0.1 M β-mercaptoethanol and detected with GAPDH antibody as loading control. Vimentin protein was performed as what we described above.
Xenografts in athymic mice
AsPC-1 cells were grown in 150 mm2 dishes to 80% confluence, harvested with 10 mM EDTA and resuspended in PBS for 107 cells/mL. A suspension of 2×106 cells in a 0.2 mL PBS was injected subcutaneously (s.c.) into the hind leg of athymic nude mice using a 27.5-gauge needle. Tumors were allowed to grow for seven days before treatment. Thirty-two nude mice with established tumors (around 100 mm3) were divided into four groups and treated with: (I) vehicle alone; (II) 12 mg/kg of 5-FU three times weekly for 4 weeks; (III) 150 µg/kg of TPL three times weekly for four weeks; (IV) TPL combined with 5-FU. Tumor size was measured three times per week with vernier calipers. The tumor volume was determined according to the formula (length × width2)/2. At the end of the experiment, the mice were killed and the tumors were removed and measured. The animal protocol was approved by Animal Care and Use Committee at Fujian Medical University.
Statistical analyses
All data are expressed as the mean ± SD of at least three determinations, unless otherwise stated. The data were analyzed by ANOVAs and t-tests. The results were considered significant at P<0.05.
Results
Effect of TPL or 5-FU alone on the growth of AsPC-1 cells
The results of SRB assay showed TPL or 5-FU alone significantly inhibited AsPC-1 cell proliferation in a dose- and time-dependent manner (P<0.01). As shown in Figure 1A, 5-FU at 13 ug/mL had an inhibitory rate of 37.15% and at 1,300 ug/mL the inhibitory rate was 81.79% after 48 hours treatment. The results (Figure 1B) showed that the inhibitory rate was 40.68% at 9 ng/mL of TPL and increased to 78.43% at 36 ng/mL of TPL after 48 hours treatment.

Effect of TPL combined with 5-FU on the growth of AsPC-1 cells
To define an optimal dose of TPL for the combination with 5-FU, the human AsPC-1 pancreatic cancer cells in 96 well plates were treated with different dose of 5-FU alone or combined with 9 ng/mL of TPL for 2 days and the cells viability was measured by SRB. As shown in Figure 2A, 13 ug/mL of 5-FU alone can inhibit 37.15% AsPC-1 cells; however, when 9 ng/mL TPL was added, the inhibitory rate increased to 78.52%, suggesting that the combination of TPL with 5-FU achieved a better treatment effect. To further determine this synergistic effect, we analyzed the interaction between TPL and 5-FU using NDST-21 software (18-20), a widely used statistical software to analyze and quantify the synergic effect of drugs in China. The results (Figure 2B) showed that he combined application of TPL plus 5-FU in AsPC-1 cells with different concentration has an obvious synergy. Table 1 indicated that we calculated two-drug combination index (CI) via the inhibition rate (0.1–1). When CI >1.2, two drugs have synergy; when 0.8≤ CI ≤1.2, two drugs are additive; when CI <0.8, two drugs have antagonism.
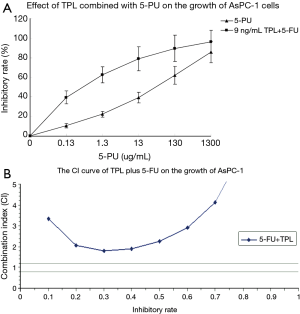
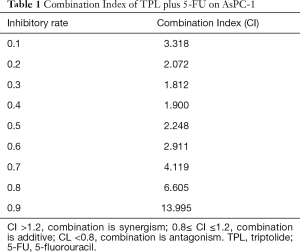
Full table
Combination of TPL and 5-FU enhances the apoptosis of AsPC-1 cells via inhibition of vimentin expression
To characterize the apoptosis induced by TPL and 5-FU, Annexin V/PI staining was used for the quantification of pancreatic cancer cells treated by TPL, 5-FU alone or combination for 48 hours. Assessed with flow cytometry, while the cells stained positive with Annexin V/PI were 11.39% or 37.18% with 5-FU or TPL treatment, the combined treatment induced apoptotic cells up to 61.99% (Figure 3A). Our data demonstrated that TPL and 5-FU alone significantly promoted apoptosis compared with group control (P<0.01,) and the combined effects were stronger than the single treatment (P<0.01, Figure 3A). The key apoptotic molecules in common path were also studied. The caspase-3 activity was enhanced to reach the peak at the 48th hour and last to the 72nd hour after the combined treatment (Figure 3B), which was consistent with the increased cleaved form of caspase-3 as demonstrated by western blot (Figure 3C). We used two-dimensional gel electrophoresis to screen out vimentin protein involved in the mechanism of combined treatment (data not shown). To further explore the role of vimentin in the enhanced apoptosis on AsPC-1 cells, vimentin protein expression in AsPC-1 cells with different treatments was determined by western blot. The results showed TPL alone decreased vimentin expression compared to 5-FU alone, but TPL plus 5-FU significantly decreased more compared to TPL alone (Figure 3D). The data suggest the synergistic effect of TPL combined with 5-FU might get through inhibiting vimentin expression to induce apoptosis.
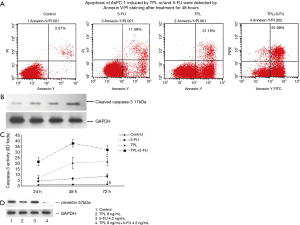
Combination of TPL with 5-FU enhances therapeutic effect in vivo
To determine whether the combination effect of pancreatic cancer cells in vitro was translatable in vivo, the AsPC-1 cells injected into the hind leg of nude mice to establish a xenograft model. The tumor growth curve measured starting on day 7 (Figure 4A) showed that the TPL and 5-FU alone could control the tumor progression (P<0.01), but the combined treatment was even more effective. At the end of the experiment, the mice were sacrificed and the tumors were weighted. The tumors in vehicle mock treated group reached a mean of 1.80 gram whereas 0.7–1.0 gram in the single treatment and nearly 0.4 gram in the combined treatment (Figure 4B). These results show that the antitumor effect of TPL combined with 5-FU was superior to the effect of the drugs when used individually.
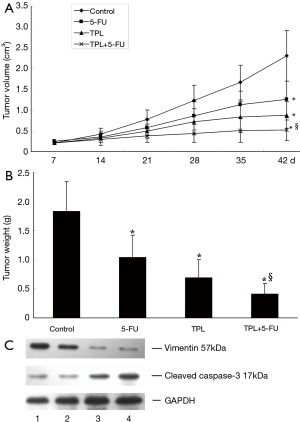
To determine whether apoptosis was involved in TPL-enhanced regression of AsPC-1 tumors, the cleaved caspase-3 was detected by western blot in tumor sections from all groups. As shown in Figure 4C, TPL or 5-FU alone could produce some cleaved caspase-3, but the combined treatment had an increased cleaved caspase-3 expression, indicating that the TPL-enhanced anti-tumor effect was related with induction of apoptosis, which was consistent with the finding in vitro. The vimentin proteolysis occurs secondary to caspase pathway activation. Vimentin proteolysis releases potential pro-apoptotic proteolytic fragments that can markedly enhance apoptosis (19,20). Therefore, vimentin protein in tumor tissues was detected by western blot (Figure 4C). The results show that dual treatment decreased vimentin expression significantly compared to single treatment, which was consistent with the finding in vitro. Our data suggest that the enhanced killing effect of dual treatment is related to the vimentin-dependent apoptotic pathway.
Discussion
For unresectable advanced pancreatic cancer, the main treatment is chemotherapy; chemotherapy is the main treatment to remit symptoms and provide some inoperable patients with the chance of surgery (21).
5-FU was first reported for chemotherapy in the treatment of pancreatic cancer (22). By blocking the synthesis of deoxy uridine nucleotide into DNA and RNA, 5-FU interferes tumor cell cycle and induces apoptosis of tumor cells. However, only 20–30% of the patients’ symptoms can be effectively improved. The effect is not ideal and also accompanied with systemic adverse reactions (22). Clearly, the combination of chemotherapy is a promising strategy (22). In seeking of new agents, we found that TPL at an extremely low dose (9 ng/mL in vitro and 0.25 mg/kg in vivo) could exert strong inhibitory effect on the AsPC-1 pancreatic cancer cells and TPL-induced apoptosis has been examined in several types of cells (23-25).
Due to the differences of cell-intrinsic suicide machinery among different cells, the sensitivity of cells to the apoptotic stimuli varies with cell lines (24-26). Among the three cell lines of pancreatic cancer, we found that AsPC-1 was less sensitive to chemo-drug including gemcitabine and 5-FU than other cell lines (data not shown). Therefore, AsPC-1 was chosen as the target cells for the treatment of TPL combined with 5-FU. The in vitro data showed the extent of apoptosis was greatly enhanced by the combined treatment compared to TPL or 5-FU alone in this drug-resistant cell line.
It was reported that TPL combined with 5-FU had a strong synergistic effect and significantly enhanced the inhibitory effect of 5-FU on the proliferation in colon cancer cell HT-29 (27). Other studies reported that TPL enhanced 5-FU-induced apoptosis in hepatocellular carcinoma cell line SMMC 7721 (28). However, the specific target of TPL-enhanced apoptosis of tumor cells is not clear.
In our study, we investigated the inhibitive effect of TPL combined with 5-FU on human pancreatic cancer cells in vitro and in vivo. We found that TPL or 5-FU alone inhibited significantly the proliferation of AsPC-1 cells in a dose- and time-dependent manner. The combined effect of TPL and 5-FU on the growth of human pancreatic cancer cells was much stronger than that of TPL or 5-FU alone in vitro and in vivo and the combined effect was synergistic at lower concentrations. We believe that the potential to translate this novel combination therapy into clinical trial is possible since 5-FU is a standard chemotherapy drug and TPL has been used to treat a number of tumors in pre-clinical trial (24-26). The use of this novel therapeutic strategy is expected to control the devastating pancreatic cancer and prolong the patient’s life.
In the process of investigating the mechanism of this synergistic effect, we found vimentin expression might be correlated with the enhanced mechanism. Vimentin is the gene encoding the intermediate filament protein. It is involved in cellular structure and integrity, which is a Wnt activity-targeted gene expressed in normal mesenchymal cells (29,30). Vimentin influences cell shape and motility in the process of epithelial-to-mesenchymal transition (EMT) that occurs during metastasis (29-31). The role of vimentin in apoptosis has been elucidated. Vimentin undergoes rapid proteolysis upon diverse pro-apoptotic stimuli, including Fas, TRAIL, TNF-α, and tamoxifen (31). This vimentin proteolysis occurs secondary to caspase pathway activation, resulting in cleavage of vimentin by multiple caspases at several sites (Asp85, Asp259, and Asp429) (16). It is possible that vimentin proteolysis and collapse contribute to many of the morphological manifestations of apoptosis, including cellular rounding, nuclear condensation, and packaging of the debris of dying cells into apoptotic bodies (17). Furthermore, Vimentin proteolysis releases potential pro-apoptotic proteolytic fragments that can markedly enhance apoptosis (16,17). This might explain the effect of vimentin in enhancing the apoptosis of AsPC-1 cells induced by TPL combined with 5-FU. Therefore, vimentin might be a relevant target for cancer therapeutic interventions. Since vimentin is overexpressed in a number of cancers, we think it is a potential cancer therapeutic target. Besides its known role in affecting cell structure, integrity, and reaction to stress, vimentin may influence cell signaling. It deserves further investigation.
In summary, TPL or 5-FU alone can inhibit the proliferation of AsPC-1 cells and induce cells apoptosis, and their combination has a synergistic effect on killing AsPC-1 cells. The synergistic mechanism is related with vimentin expression which may play the key role in inducing apoptosis on human pancreatic cancer cell line AsPC-1. However, the complete apoptotic signaling of combined treatment is still unclear and needs to be investigated further.
Acknowledgments
Funding: This work was supported by Young and Middle-aged Talents Project of Fujian Health and Family Planning Commission (2016-ZQN-54) and the Key Program of Scientific Research of FMU (09ZD014).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This article was involved in animals’ study and the animal protocol was approved by Animal Care and Use Committee at Fujian Medical University and all operations complied with the animal experiment regulations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Strobel O, Hank T, Hinz U, et al. Pancreatic cancer surgery: the new r-status counts. Ann Surg 2017;265:565-73. [Crossref] [PubMed]
- Martin RC 2nd. Management of Locally Advanced Pancreatic Cancer. Surg Clin North Am 2016;96:1371-89. [Crossref] [PubMed]
- Di Marco M, Di Cicilia R, Macchini M, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? Oncol Rep 2010;23:1183-92. (Review). [Crossref] [PubMed]
- Onesti CE, Romiti A, Roberto M, et al. Recent advances for the treatment of pancreatic and biliary tract cancer after first-line treatment failure. Expert Rev Anticancer Ther 2015;15:1183-98. [Crossref] [PubMed]
- Mujumdar N, Mackenzie TN, Dudeja V, et al. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology 2010;139:598-608. [Crossref] [PubMed]
- Carter BZ, Mak DH, Schober WD, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood 2006;108:630-7. [Crossref] [PubMed]
- Meng C, Zhu H, Song H, et al. Targets and molecular mechanisms of triptolide in cancer therapy. Chin J Cancer Res 2014;26:622-6. [PubMed]
- Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res 2007;67:9407-16. [Crossref] [PubMed]
- Yinjun L, Jie J, Yungui W. Triptolide inhibits transcription factor NF-kappaB and induces apoptosis of multiple myeloma cells. Leuk Res 2005;29:99-105. [Crossref] [PubMed]
- Fuchs O. Transcription factor NF-κB inhibitors as single therapeutic agents or in combination with classical chemotherapeutic agents for the treatment of hematologic malignancies. Curr Mol Pharmacol 2010;3:98-122. [Crossref] [PubMed]
- Panichakul T, Intachote P, Wongkajorsilp A, et al. Triptolide sensitizes resistant cholangiocarcinoma cells to TRAIL-induced apoptosis. Anticancer Res 2006;26:259-65. [PubMed]
- Jacobson BA, Chen EZ, Tang S, et al. Triptolide and its prodrug minnelide suppress Hsp70 and inhibit in vivo growth in a xenograft model of mesothelioma. Genes Cancer 2015;6:144-52. [PubMed]
- Xu B, Guo X, Mathew S, et al. Triptolide simultaneously induces reactive oxygen species, inhibits NF-kappaB activity andsensitizes 5-fluorouracil in colorectal cancer cell lines. Cancer Lett 2010;291:200-8. [Crossref] [PubMed]
- Ghadban T, Dibbern JL, Reeh M, et al. HSP90 is a promising target in gemcitabine and 5-fluorouracil resistant pancreatic cancer. Apoptosis 2017;22:369-80. [Crossref] [PubMed]
- Wang C, Liu B, Xu X, et al. Toward targeted therapy in chemotherapy-resistant pancreatic cancer with a smart triptolide nanomedicine. Oncotarget 2016;7:8360-72. [PubMed]
- Byun Y, Chen F, Chang R, et al. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ 2001;8:443-50. [Crossref] [PubMed]
- Zhu QS, Rosenblatt K, Huang KL, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 2011;30:457-70. [Crossref] [PubMed]
- Huang XH, Xie HT, Sun RY. Introduction and application of new edition of software of the new drug statistical treatment (NDST-21). Chin J Clin Pharmacol Ther 2002;3:141-9.
- Cheng NN, Sun RY. Introduction of new statistical software for the data analisis of new drug treatment (NDST). Chin J Clin Pharmacol Ther 1997;2:137-8.
- Lv Y, Zhang Z, Gong Z, et al. Determination and pharmacokinetics of ergometrine maleate in rabbit blood with on line microdialysis sampling and fluorescence detection. J Pharm Biomed Anal 2005;38:29-33. [Crossref] [PubMed]
- Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol 2015;21:9297-316. [Crossref] [PubMed]
- Li D, O'Reilly EM. Adjuvant and Neoadjuvant Therapy for Pancreatic Cancer. Surg Oncol Clin N Am 2016;25:311-26. [Crossref] [PubMed]
- Wang W, Yang S, Su Y, et al. Enhanced antitumor effect of combined triptolide and ionizing radiation. Clin Cancer Res 2007;13:4891-9. [Crossref] [PubMed]
- Wang W, Li X, Sun W, et al. Triptolide triggers the apoptosis of pancreatic cancer cells via the downregulation of Decoy receptor 3 expression. J Cancer Res Clin Oncol 2012;138:1597-605. [Crossref] [PubMed]
- Frese S, Pirnia F, Miescher D, et al. PG490-mediated sensitization of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires activation of ERK2. Oncogene 2003;22:5427-35. [Crossref] [PubMed]
- Wang X, Matta R, Shen G, et al. Mechanism of triptolide-induced apoptosis: Effect on caspase activation and Bid cleavage and essentiality of the hydroxyl group of triptolide. J Mol Med (Berl) 2006;84:405-15. [Crossref] [PubMed]
- Tang XY, Zhu YQ, Tao WH, et al. Synergistic effect of triptolide combined with 5-fluorouracil on colon carcinoma. Postgrad Med J 2007;83:338-43. [Crossref] [PubMed]
- Li Y, Hu S. Triptolide sensitizes liver cancer cell lines to chemotherapy in vitro and in vivo. Panminerva Med 2014;56:211-20. [PubMed]
- Gilles C, Polette M, Mestdagt M, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res 2003;63:2658-64. [PubMed]
- Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 2010;24:1838-51. [Crossref] [PubMed]
- Lazarova DL, Bordonaro M. Vimentin, colon cancer progression and resistance to butyrate and other HDACis. J Cell Mol Med 2016;20:989-93. [Crossref] [PubMed]


