
Management of BRCA mutation carriers
Introduction
An inherited pathogenic mutation in BRCA genes causes an enhanced lifetime possibility of manifesting breast cancer (BC) and ovarian cancer (OC). Operative prophylactic strategies have been performed making genetic counselling an indispensable part of the management of these patients. Approximately 5% of all BC and 15–20% of hereditary BC depends on BRCA1 or BRCA2 gene mutations. According to current evidence, 55–65% of BRCA1mut and 45% of BRCA2mut carriers will manifest BC by 70 years. Nowadays, BRCAmut BC could receive new therapeutic approaches. We review the current effective risk-reducing therapy and we show the future research prospects.
BC and mutation: surgery
Risk-reducing mastectomy (RRM)
Bilateral RRM seems to decrease the estimated lifetime risk of developing BC by more than 90% among BRCA mutation patients (1-10).
Domchek et al. (1) found that RRM decreased the lifetime possibility of manifesting BC in BRCAmut. After 3 years of follow-up period (FUP), no women treated with RRM manifested a BC, compared to 7% of patients without RRM who experienced a BC. Furthermore, risk-reducing salpingo-oophorectomy (RRSO) decreases the possibility of BC in both BRCA1 (HR =0.63; 95% CI: 0.41–0.96) and BRCA2 (HR =0.36; 95% CI: 0.16–0.82) mutation carriers with no personal history of BC. A risk reduction effect in BC among BRCA1 patients with RRSO before 50 years (HR =0.51; 95% CI: 0.32–0.82) has been noticed, but no advantages were observed in those with RRSO after 50 years (HR =1.36; 95% CI: 0.26–7.05) (1).
Rebbeck et al. (2) found that bilateral prophylactic mastectomy (BPM) decreased the risk of BC in patients without prior RRSO by 90% (HR =0.09; 95% CI: 0.02–0.38); only 2 patients out of 105, with prior RRM, experienced a BC (mean FUP of 5.3 years). In women with concurrent or prior RRSO, the BC risk-reduction was more considerable (HR =0.05; 95% CI: 0.01–0.22).
Hartmann et al. (3), in a retrospective cohort study, found that bilateral RRM decreased the incidence and mortality of BC in both the moderate- and high-risk groups by 90%. These advantages must be evaluated carefully; current studies have advised that much of the gain of bilateral salpingo-oophorectomy (BSO) on BC risk might be derived to selection bias rather than a true gain (11).
Mortality benefit
Two recent studies (mean FUP of 13.3 and 8.5 years, respectively) evaluated the impact of bilateral RRM in BRCA carriers on mortality (6,7). Ingham et al. found that BPM wasn’t significantly linked with reduced death for all causes (HR =0.226; 95% CI, 0.05–1.016). Heemskerk-Gerritsen et al. found that BC specific mortality was also not significantly decreased (HR =0.29; 95% CI: 0.03–2.61). The BC reduction-risk with bilateral RRM, nevertheless, has not brought benefits in terms of survival, since longer follow-up are needed.
In a decision analysis, Schrag et al. (12) correlated RRM and RRSO with no risk-reducing strategies among BRCA mutation carriers. They created hypothetical cohorts of patients using early estimates of the cumulative risk of BC among BRCAmut to calculate the effect of prophylactic strategies on survival. The authors calculated this risk by a Markov model. This study found a considerable gain in life expectancy with RRM than RRSO. Anyway a gain in life expectancy of 4 years does not mean that every patient will earn 4 years of life.
Contralateral prophylactic mastectomy (CPM)
BRCA carriers have an enhanced rate of contralateral breast cancer (CBC). In a meta-analysis of 11 studies (7 cohort and 4 case-control studies), including 807 carriers and 3,163 non-carriers, Valachis et al. (13) asserted that the rate of CBC for BRCA carriers was 23.7% (95% CI: 17.6–30.5%) while for non-carriers was 6.8% (95% CI: 4.2–10%). BRCA carriers had an enhanced rate of CBC compared with controls [response rate (RR) =3.56; 95% CI: 2.50–5.08; P<0.001]. BRCA1mut carriers presented an enhanced probability of manifesting CBC compared to BRCA2mut (21.1% for BRCA1mut vs. 15.1% for controls). In this meta-analysis only RRSO (RR =0.52; 95% CI: 0.37–0.74) and age >50 years old seem to reduce, statistically, the risk for CBC. Moreover, a recent study (14) asserts that women with a BC diagnosis before 41 years showed a 10-year possibility of manifesting a CBC of 23.9%, while those with a diagnosis between 41 and 49 years had a risk of 12.6%.
Mortality benefit
There are limited data about the efficacy of CPM to improve survival in BRCA1/2 carriers. Two studies (15,16) (mean FUP of 4.3 and 3.4 years, respectively) reported data on breast cancer specific survival (BCSS) in BRCA carriers after RRM vs. therapeutic mastectomy while only one (16) on overall survival (OS). There was no disparity in BCSS between BRCA carriers who underwent CPM and those who didn’t (HR =0.78; 95% CI: 0.44–1.39; P=0.40). A single study (16) showed a 94% OS for RRM group vs. 77% for controls (P=0.03). Anyway, if adjusted for other factor as RRSO, carriers in the first group didn’t reach an enhancement in terms of survival than those in the second group (P=0.14).
Metcalfe et al. (17) in a recent retrospective analysis conclude that BRCA mutated (BRCAmut) women treated for early stage BC who underwent bilateral mastectomy presented an enhanced risk for death for BC compared to women who underwent unilateral mastectomy. At 20 years the survival rate for patients with contralateral mastectomy was 88% vs. 66% for those who didn’t. After controlling for age at diagnosis and therapy, contralateral mastectomy showed a decreased risk of death for BC by 48% (HR =0.52; 95% CI: 0.29–0.93; P=0.03).
Breast-conserving therapy (BCT) in BRCAmut
Breast conserving surgery (BCS) followed by radiotherapy (RT) is currently considered the gold standard approach in early-stage sporadic BC (18,19). There is a paucity data about the use of a conservative strategy in BRCA carriers.
Ipsilateral breast recurrence (IBR), CBC and survival after BCT in BRCAmut
The rate of ipsilateral recurrence following BCT is higher in BRCA mutation patients. IBR is also higher in BRCAmut treated with BCT than those treated with mastectomy (Table 1).
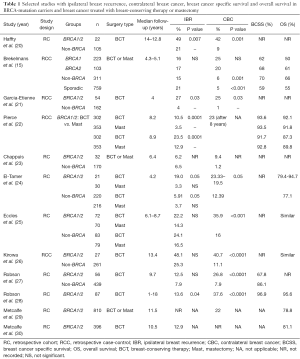
Full table
In 2002, Haffty et al. (20) reported high rates of IBR and CBC following BCT in BRCAmut. From 1975 to 1998, they studied 290 women (105 with sporadic BC and 22 with genetic predisposition) with BC before 42 years treated with BCT. After 12 years of FUP, BRCA carriers showed an increased rate of IBR (identified as second BC in mostly cases) (49% vs. 21%, P=0.007) and CBC (42% vs. 9%, P=0.001) than controls. However, there are bias in this study: none of the three women of genetic group with positive oestrogen receptor (ER)/progesterone receptor (PR) status were treated with adjuvant hormonal therapy; dead patients were excluded from this database; 19 patients (14.9%) had no axillary surgery despite only 10 cases (5.8%) are tumour in situ; surgical margins status were unknown in 50% of BRCA mutation carriers. No data on BCSS and OS were reported.
In 2007, Brekelmans et al. (15), between 1980 and 2004, studied 103 BRCA2, 223 BRCA1 and 311 non-BRCA BC women. The IBR rate was the same between carriers and non-carriers following BCT (16% and 17% for BRCA1 and BRCA2, respectively; 15% and 21% for non-BRCA hereditary BC and sporadic BC patients, respectively). The 10-year actuarial risk to develop a CBC was higher in BRCAmut (25% for BRCA1mut and 20% for BRCA2mut) compared with non-BRCAmut hereditary BC and sporadic BC patients (6% and 5%, respectively; P≤0.001 compared with BRCA2 associated cancers). OS and BCSS were the same between groups. On multivariate analysis, the administration of adjuvant chemotherapy and RRSO, but not CPM (or bilateral mastectomy), were the only prognostic factors for BCSS in BC BRCA-related.
In 2009, Garcia-Etienne et al. (21), between 1994 and 2007, studied 54 BRCA carriers with BC and 162 sporadic BC who underwent BCT (4 years of follow-up). After 10 years IBR and CBC rates were 27% and 25% for carriers, 4% and 1% for sporadic cancer (P=0.03), respectively. We need to consider that 8 of 11 (73%) women with IBR (n=6) or CBC (n=5) performed genetic test after breast recurrence.
In 2010, Pierce et al. (22), studied 655 BRCA carriers with BC who underwent breast conservative surgery plus RT (n=302) or mastectomy (n=353). Estimated IBR rates were greater at all-time points in patients who underwent breast conservative therapy than those who underwent mastectomy: 4.1% vs. 1.4% at 5 years, 10.5% vs. 3.5% at 10 years and 23.5% vs. 5.5% at 15 years. Hormonal therapy seems to reduce the rate of IBR principally in BRCA2 carriers (BRCA: P=0.08; BRCA1: P=0.13). CBC rates was similar in patients treated with or without adjuvant RT (P=0.44), supposing that RT has no influence on CBC. The 10 and 15 years risk of BCSS after BCT was 93.6% and 91.7%, while after mastectomy was 93.5% and 92.8%, respectively (P=0.85). The 10 and 15 years risk of OS after BCT was 92.1% and 87.3%, while after mastectomy was 91.8% and 89.8%, respectively (P=0.73). The evidence of an infiltrating lobular cancer (HR =4.3; P=0.01) and the growth of a CBC (HR =2.5; P=0.02) were linked to breast cancer-specific mortality (BCSM). About OS the growth of OC was related to enhanced rates of death (HR =5.0; P=0.0001).
In 2011, Metcalfe et al. (30), between 1975 and 2008, studied 396 BRCAmut BC treated with BCT. The 5- and 10-year risk of IBR was 5.8% (95% CI: 3.2–8.4%) and 12.9% (95% CI: 8.7–17.1%). Three factors reduced the rate of IBR: adjuvant chemotherapy (70.2%, RR =0.45; 95% CI: 0.24–0.84; P=0.01), RT (87.4%, RR =0.28; 95% CI: 0.12–0.63; P=0.002), salpingo-oophorectomy (33.3%, RR =0.33; 95% CI: 0.13–0.81; P=0.02).
In 2011, Metcalfe et al. (29), between 1975 and 2008, studied the CBC risk in 810 BRCAmut BC who underwent BCT or mastectomy. In total, after a median FUP of 11.1 years, 149 (18.4%) manifested a CBC, with a median FUP of 5.7 years (range, 0.2–15 years) between first BC and CBC. The 5-, 10- and 15-year risk of CBC was 13.1% (95% CI: 10.3–15.9%), 22.0% (95% CI: 19.2–26.8%), and 33.8% (95% CI: 28.6–39.0%), respectively. The annual risk was 2.1%. Carriers with BC diagnosed at age 50 years or older manifested a decreased possibility to develop a CBC than those diagnosed at age 40 years or younger (RR =0.47; 95% CI: 0.47–0.82; P=0.008). Women who underwent salpingo-oophorectomy had a decreased risk of CBC, than those with intact ovaries (RR =0.48; 95% CI: 0.27–0.82; P=0.002). This reduction was significant in those diagnosed at 50 years or younger (RR =0.39; 95% CI: 0.23–0.67; P=0.0006).
Valachis et al. (13) identified two protective factors against IBR in BRCAmut: the administration of adjuvant chemotherapy (RR =0.51; 95% CI: 0.31–0.84), and RRSO (RR =0.42; 95% CI: 0.22–0.81).
Prophylactic bilateral RRSO
RRSO is recommended for BRCAmut by the age of 35 to prevent BC and OC, while no evidence are seen about its influence on survival in patients with BC associated to a BRCA mutation (31-33).
Finch et al. (34) found that RRSO decreased by 77% the all-cause mortality before the age of 70 in BRCA carriers. This reduction was evident in patients with prior BC (HR =0.31; 95% CI: 0.24–0.39) especially in BRCA1mut (HR =0.21; 95% CI: 0.12–0.37). Domchek et al. (1) documented an OR of 0.35 (95% CI: 0.19–0.67) for salpingo-oophorectomy and BCSM among patients with prior BC.
In another retrospective study of the 676 patients with stage I–II BC, 345 performed salpingo-oophorectomy after BC diagnosis and 331 preserved intact ovaries. The 20-year OS was 77.4%. The adjusted HR for death for BC in patients with RRSO was 0.38 for BRCA1 carriers (95% CI: 0.19–0.77; P=0.007) and 0.57 for BRCA2 carriers (95% CI: 0.23–1.43; P=0.23). The HR for BCSM was 0.76 (95% CI: 0.32–1.78; P=0.53) in patients with ER-positive BC and 0.07 (95%CI: 0.01–0.51; P=0.009) in patients with ER-negative BC. We can conclude that salpingo-oophorectomy decreases mortality in patients with BRCA1-associated BC. Women with BRCA1 ER-negative BC should undergo salpingo-oophorectomy after diagnosis (35).
BC and BRCA mutation: therapy
BRCA mutated locally advanced breast cancer (LABC) and metastatic breast cancer (MBC): clinical management and new evidence
Patients harbouring BRCA1 or BRCA2 (BRCA1/2) gene mutations are responsible for approximated 5% of all BC (36) and approximately 15–20% of hereditary BCs (37). According to recent assessments, 55–65% of BRCA1 carriers and around 45% BRCA2 carriers will develop BC by the age of 70 years (38). Actually, there are no definitive guidelines on the optimal chemotherapy for these patients, but there is increasing evidence of enhanced sensitivity to specific drugs in this patient population.
BRCAmut could show a pronounced sensitivity to platinum-based antineoplastic drugs probably due to their DNA-damaging mechanism of action. There is a paucity of data about the clinical efficacy of these agents in BRCA carriers in the metastatic setting (39), such as in triple negative breast cancer (TNBC) (40). In neoadjuvant setting, series reported a RR increase of 26% in positive family history and 23% in BRCAmut patients with platinum-based treatment. In the metastatic setting, prospective studies reported RR in a range from 10% to 40% when cisplatin or carboplatin were included (41,42).
Tamoxifen
In prospective trials, tamoxifen decreased the risk of BC and CBC in patients at high risk (43,44). An analysis of the National Surgical Adjuvant Breast and Bowel Project identified 19 BRCA1/2 mutations among 288 women with BC (45). Five of 8 BRCA1 carriers had taken tamoxifen vs. 3 of 11 BRCA2 carriers. Although the sample size was small, this analysis showed a decrease in BC incidence by 62% for BRCA2 carriers without effective benefit in BRCA1 carriers.
Narod et al., in a case-control study, analysed the efficacy of treatment with tamoxifen and the possibility of developing a CBC in BRCAmut comparing patients with bilateral and unilateral BC (46). Sixty-four BRCA1 mutation carriers (13%) used tamoxifen vs. 39 BRCA2 mutation carriers (33%). This difference was expected because BRCA1-associated BCs are typically ER-negative and BRCA2-associated BCs are commonly ER-positive. Tamoxifen seems to be protective against CBC, with OR of 0.38 in BRCA1mut and 0.63 for BRCA2mut. The combined risk reduction for BRCA mutation carriers was 50%. This study also revealed a decrease in CBC in women who underwent RRSO (OR of 0.42), similar to the risk-reduction with tamoxifen.
LABC
A German group presented data from GeparSixto related to BRCA mutation and family history of BC or OC. Mutation in BRCA1/2 genes was evaluated in 94% of patients with TNBC from 315. An increase of 26.7% in polymerase chain reaction (pCR) was observed with addition of carboplatin in patients with a positive relatives’ anamnesis for BC or OC despite absence of BRCA mutation (pCR: 49%). In patients’ gBRCA1/2 mut, the increase in pCR rate was 23.2% (pCR: 55%). Therefore, mutations in BRCA1/2 and family history for BC or OC are strong predictors for improvement in pCR rates after carboplatin in TNBC (47).
An adaptive study published in 2016 from Rugo et al. (I-Spy2), matched experimental regimen with veliparib in addition to carboplatin with responding cancer subtype (gBRCAmut). This study revealed that the addiction of veliparib and carboplatin to usual chemotherapy produced higher rates of PCR compared to usual chemotherapy regimens (pCR: 51% vs. 26%) (48).
Studies are ongoing with talazoparib (NCT02282345), olaparib (PARTNER NCT03150576) and veliparib (NCT01818063).
MBC
In a phase III randomized control trial (RCT) who compared carboplatin and docetaxel in BRCAmut metastatic or recurrent LABC, overall RR was 68% in those treated with carboplatin vs. 33.3% in those with docetaxel with a progression free survival (PFS) of 6.8 vs. 3.1 months, respectively (49).
The TBCRC009 study evaluated the efficacy of cisplatin 75 mg/mq d1q21 or carboplatin (AUC 5) d1q21, according to clinical choice in pre-treated MBC. RR was 25.6% (95% CI: 16.8–36%) and was higher with cisplatin (32.6%) compared with carboplatin (18.7%). RR was 54.5% BRCA1/2 carriers (n=11) (50).
Keeping in mind current evidence, most of the ABC2 panel promoted the inclusion of platinum-containing regimens in the treatment of BRCA carriers pre-treated with anthracyclines and taxanes and proved to be endocrine-resistant (51).
A promising area of clinical research is the investigation of poly (ADP-ribose) polymerase inhibitors (PARPi) in treating BRCA MBC
Olaparib
In a phase II RCT, 54 recurrent BRCAmut LABC, received olaparib administered orally. In 27 women, who got the maximum tolerated dose of 400 mg twice daily, the overall response rate (ORR) was 41% (52). A following multicenter phase II RCT studied the efficacy of olaparib in 298 heavily pre-treated BRCA1/2 mutated with recurrent solid cancers. The ORR was 12.9% (8 of 62 BC patients), and disease stabilization for at least 8 weeks was showed in 47% of them. The ORR was better for women with no previous exposition to platinum-based antineoplastic drugs (20% vs. 9.5%) (53). Gelmon et al. (54), in a study with olaparib monotherapy, hypothesized that heavy pre-treatment could decrease the response to olaparib in TNBC associated to BRCAmut. Recently, in American Society of Clinical Oncology (ASCO) meeting 2017, Robson et al. presented OlympiAD trial compared olaparib as a single agent vs. chemotherapeutic agents of physician’s choice in late-line MBC. The results showed a clinical and statistical benefit in delaying PFS with olaparib. Three hundred and two patients were randomized of whom 205 received olaparib and 91 received TPC (6 TPC patients were not treated). PFS was significantly longer in those who received olaparib vs. TPC (HR =0.58; 95% CI: 0.43–0.80; P=0.0009; 7.0 vs. 4.2 months, respectively). Objective response rate was 59.9% and 28.8% in olaparib and TPC arms, respectively (55).
Veliparib
In a phase II RCT presented at the ASCO Annual Meeting 2014, 44 BRCAmut MBC received oral veliparib at 400 mg twice daily. When a progression was noticed, it was switched to oral veliparib (150 mg orally twice daily) plus carboplatin. Forty-one women (out of 44) were treated. The partial response rate (PR) was 17% (2 out of 12) in BRCA1mut and 23% (3 out of 13) in BRCA2mut evaluated at least 4 cycles of follow-up (56).
Niraparib
Studies with niraparib are outstanding. BRAVO is a phase III multicenter RCT that enrols gBRCAmut women with HER2 negative BC who received niraparib vs. physician’s choice (capecitabine, eribulin, gemcitabine, or vinorelbine), excluding women with progressive disease during previous platinum-based therapy (57).
Talazoparib
The ABRAZO phase II trial evaluates the efficacy of talazoparib in BRCAmut LABC or MBC women. Patients were randomized in two cohorts: women with MBC responsive to PARPi or women with MBC treated with non-platinum cytotoxic regimens (58). The EMBRACA phase III trial comparing talazoparib 1 mg daily in 21-day cycles vs. physician’s choice (eribulin or capecitabine or gemcitabine or vinorelbine) (59).
New chemotherapeutics and activity in BRCAmut BC
About other drugs, new evidence is due to the efficacy of trabectedin and lurbinectedin. In heavily pre-treated MBC patients with gBRCA mutations, trabectedin showed a PR of 17% (6 out of 35) and a mean PFS of 3.9 months (60). In the same way, a phase II RCT showed a PR of 14% and mean PFS of 3.3 months (61).
The ORR in 17 BRCAmut MBC women treated with lurbinectedin was 41% with a mean response period of 5 months, compared with 9% and 3.3 months of an unselected cohort. An exploratory analysis of 17 women showed a high ORR in BRCAmut with no prior exposition to platinum based antineoplastic drugs (64%) (62). At the European Society of Medical Oncology (ESMO) 2016, Balmana et al. presented a phase II RCT in which 54 BRCAmut MBC received lurbinectedin 7 mg, decreased to 3.5 mg/m2 intravenous (IV) after 3 weeks. The ORR was 39% and 44% in 7 and 3.5 mg/m2 dosage group, respectively. More than half were pre-treated with platinum therapy. There was a higher ORR (61% vs. 26%), PFS (5.9 vs. 2.1 months) and OS (31.8 vs. 11.8 months) in BRCA2mut compared to BRCA1mut. A growing number of studies valuating this drug in women pre-treated with PARPi are underway (63).
Discussion
There are several issues to keep in mind about the management of BRCA carriers. When a BRCA carrier is identified, the type of BCS must be carefully considered and discussed. Recent, large, multicenter studies have confirmed that BCT is linked with a higher rate of IBR, but no survival advantage has been demonstrated following more radical surgery. Risk-reducing strategies of IBR in carriers who underwent BCT include the administration of adjuvant chemotherapy, RRSO and RT. We can state that the different location, the different histology and the higher FUP period between first cancer and recurrence, compared to sporadic BC, is suggestive for newly diagnosed BC rather than real IBR. The rate of CBC is higher in BRCA mutation carriers, and this is the rationale for performing CPM. RRSO can also decrease the rate of CBC in BC associated to BRCA mutation. There isn’t robust evidence to suggest that the prognosis of these patients with BC is worse than patients with sporadic BC. In addition, BRCA mutation carriers treated with BCT do not have worse overall survival. The prognosis, in fact, is driven by the biological characteristics of the tumour rather than by local treatment. Clinical guidelines about the surgical management of unilateral breast cancer for BRCAmut patients are still missing. BRCA carriers with BC need to be discussed by a multidisciplinary team keeping in mind that every surgical decision should also take into account the psychological and social impact of every procedure.
Conclusions
Risk-reducing strategies (RRM and/or RRSO) should be proposed to each BRCAmut. Data demonstrate similar long-term survival between women with early BC who underwent BCT or mastectomy. However, the enhanced risk of IBR and CBC lead many BRCA mutation patients to choose mastectomy with a CPM. If a woman refuses surgical risk-reduction strategies, she should be guided to realize adequate screening tests keeping in mind the known limitations. In mutated patients is possible to use platinum salts during treatment: carriers benefit from this therapy choice. Innovative drugs such as PARPi are an interesting innovative strategy for mutated BC and OC patients where chemotherapy is limited.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Update of Current Evidences in Breast Cancer Multidisciplinary Management”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.23). The series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” was commissioned by the editorial office without any funding or sponsorship. RM served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967-75. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst 2001;93:1633-7. [Crossref] [PubMed]
- Kaas R, Verhoef S, Wesseling J, et al. Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: very low risk for subsequent breast cancer. Ann Surg 2010;251:488-92. [Crossref] [PubMed]
- Skytte AB, Crüger D, Gerster M, et al. Breast cancer after bilateral risk-reducing mastectomy. Clin Genet 2011;79:431-7. [Crossref] [PubMed]
- Ingham SL, Sperrin M, Baildam A, et al. Risk-reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Res Treat 2013;142:611-8. [Crossref] [PubMed]
- Heemskerk-Gerritsen BA, Menke-Pluijmers MB, Jager A, et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol 2013;24:2029-35. [Crossref] [PubMed]
- Manning AT, Wood C, Eaton A, et al. Nipple-sparing mastectomy in patients with BRCA1/2 mutations and variants of uncertain significance. Br J Surg 2015;102:1354-9. [Crossref] [PubMed]
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 2015;22:370-6. [Crossref] [PubMed]
- Peled AW, Irwin CS, Hwang ES, et al. Total skin-sparing mastectomy in BRCA mutation carriers. Ann Surg Oncol 2014;21:37-41. [Crossref] [PubMed]
- Heemskerk-Gerritsen BA, Seynaeve C, Van Asperen CJ, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst 2015;107:djv033 [Crossref] [PubMed]
- Schrag D, Kuntz KM, Garber JE, et al. Decision analysis--effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med 1997;336:1465-71. [Crossref] [PubMed]
- Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat 2014;144:443-55. [Crossref] [PubMed]
- van den Broek AJ, Van T, Veer LJ, Hooning MJ, et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J Clin Oncol 2016;34:409-18. [Crossref] [PubMed]
- Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 2007;43:867-76. [Crossref] [PubMed]
- van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer 2005;93:287-92. [Crossref] [PubMed]
- Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 2014;348:g226. [Crossref] [PubMed]
- Veronesi U, Cascnelli N, Mariani L, et al. Twenty-year follow up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed earlyonset breast cancer by BRCA1/2 status. Lancet 2002;359:1471-7. [Crossref] [PubMed]
- Garcia-Etienne CA, Barile M, Gentilini OD, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol 2009;16:3380-7. [Crossref] [PubMed]
- Pierce LJ, Phillips KA, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 2010;121:389-98. [Crossref] [PubMed]
- Chappuis PO, Kapusta L, Bégin LR, et al. Germline BRCA1/2 mutations and p27(Kip1) protein levels independently predict outcome after breast cancer. J Clin Oncol 2000;18:4045-52. [Crossref] [PubMed]
- El-Tamer M, Russo D, Troxel A, et al. Survival and recurrence after breast cancer in BRCA1/2 mutation carriers. Ann Surg Oncol 2004;11:157-64. [Crossref] [PubMed]
- Eccles D, Simmonds P, Goddard J, et al. Familial breast cancer: an investigation into the outcome of treatment for early stage disease. Fam Cancer 2001;1:65-72. [Crossref] [PubMed]
- Kirova YM, Savignoni A, Sigal-Zafrani B, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat 2010;120:119-26. [Crossref] [PubMed]
- Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 2004;6:R8-17. [Crossref] [PubMed]
- Robson M, Svahn T, McCormick B, et al. Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2: a clinic-based series. Cancer 2005;103:44-51. [Crossref] [PubMed]
- Metcalfe K, Lynch HT, Ghadirian P, et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 2011;127:287-96. [Crossref] [PubMed]
- Metcalfe K, Gershman S, Lynch HT, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2011;104:1384-92. [Crossref] [PubMed]
- Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol 2005;23:7491-6. [Crossref] [PubMed]
- Finch A, Beiner M, Lubinski JHereditary Ovarian Cancer Clinical Study Group, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA 2006;296:185-92. [Crossref] [PubMed]
- ACOG Committee on Practice Bulletins. Hereditary breast and ovarian cancer syndrome. Gynecol Oncol 2009;113:6-11. [Crossref] [PubMed]
- Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 2014;32:1547-53. [Crossref] [PubMed]
- Metcalfe K, Lynch HT, Foulkes WD, et al. Effect of Oophorectomy on Survival After Breast Cancer in BRCA1 and BRCA2 Mutation Carriers. JAMA Oncol 2015;1:306-13. [Crossref] [PubMed]
- Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet 2008;124:31-42. [Crossref] [PubMed]
- Balmaña J, Díez O, Rubio IT, et al. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22:vi31-4. [Crossref] [PubMed]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007;25:1329-33. [Crossref] [PubMed]
- Byrski T, Foszczynska-Kloda M, Huzarski T, et al. Cisplatin chemotherapy in the treatment of BRCA1-positive metastatic breast cancer (MBC). J Clin Oncol 2009;27:abstr 1099.
- Koshy N, Quispe D, Shi R, et al. Cisplatin-gemcitabine therapy in metastatic breast cancer: improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast 2010;19:246-8. [Crossref] [PubMed]
- Liu M, Mo QG, Wei CY, et al. Platinum-based chemotherapy in triple-negative breast cancer: A meta-analysis. Oncol Lett 2013;5:983-91. [Crossref] [PubMed]
- Isakoff SJ. Triple-negative breast cancer: Role of specific chemotherapy agents. Cancer J 2010;16:53-61. [Crossref] [PubMed]
- Fisher B, Constantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88. [Crossref] [PubMed]
- . Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 1998;351:1451-67. [Crossref] [PubMed]
- King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA 2001;286:2251-6. [Crossref] [PubMed]
- Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet 2000;356:1876-81. [Crossref] [PubMed]
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747-56. [Crossref] [PubMed]
- Rugo HS, Olopade OI, DeMichele A, et al. Adaptive Randomization of Veliparib–Carboplatin Treatment in Breast Cancer. N Engl J Med 2016;375:23-34. [Crossref] [PubMed]
- Tutt A, Ellis P, Kilburn L, et al. The TNT trial: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). Cancer Res 2015;75:abstr S3-01.
- Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 2015;33:1902-9. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014;23:489-502. [Crossref] [PubMed]
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010;376:235-44. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852-61. [Crossref] [PubMed]
- Robson ME, Im SA, Senkus E, et al. OlympiAD: Phase III trial of olaparib monotherapy versus chemotherapy for patients (pts) with HER2-negative metastatic breast cancer (mBC) and a germline BRCA mutation (gBRCAm). J Clin Oncol 2017;35:LBA4. [Crossref]
- Somlo G, Frankel PH, Luu TH, et al. Phase II trial of single agent PARP inhibitor ABT-888 (veliparib [vel]) followed by postprogression therapy of vel with carboplatin (carb) in patients (pts) with stage BRCA-associated metastatic breast cancer (MBC): California Cancer Consortium trial PHII-96. J Clin Oncol 2014;32:abstr 1021.
- ClinicalTrials.gov NCT01905592. A phase III trial of niraparib versus physician’s choice in HER2 negative, germline BRCA mutation-positive breast cancer patients (BRAVO). 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01905592
- Turner NC, Balmaña J, Fasching PA, et al. ABRAZO: An international phase 2 (2-stage, 2-cohort) study of the oral PARP inhibitor talazoparib (BMN 673) in BRCA mutation subjects with locally advanced and/or metastatic breast cancer. Cancer Res 2016;76:abstr OT1-03–17.
- Litton JK, Blum JL, Im YH, et al. A phase 3, open-label, randomized, parallel, 2-arm international study of the oral PARP inhibitor talazoparib (BMN 673) in BRCA mutation subjects with locally advanced and/or metastatic breast cancer (EMBRACA). Cancer Res 2016;76:OT1-03-16.
- Delaloge S, Wolp-Diniz R, Byrski T, et al. Activity of trabectedin in germline BRCA1/2-mutated metastatic breast cancer: results of an international first-in-class phase II study. Ann Oncol 2014;25:1152-8. [Crossref] [PubMed]
- Tedesco KL, Blum JL, Goncalves A, et al. Final results of a phase II trial of trabectedin (T) in triple-negative, HER2-positive, and BRCA1/2 germ-line-mutated metastatic breast cancer (MBC) patients (pts). J Clin Oncol 2011;29:abstr 1125.
- Balmaña J, Cruz C, Garber J, et al. Lurbinectedin (PM01183) activity in BRCA1/2-associated or unselected metastatic breast cancer. Interim results of an ongoing phase II trial. Cancer Res 2015;75:abstr P3-13-01.
- Balmana J, Cruz C, Arun BK, et al. Anti-tumor activity of PM01183 (lurbinectedin) in BRCA1/2- associated metastatic breast cancer patients: Results of a single-agent phase II trial. Ann Oncol 2016;27:68-99. [Crossref] [PubMed]


