Evidence-based usefulness of integrative therapies in breast cancer
Introduction
Integrative oncology is a global strategy that incorporates complementary therapies to mainstream care, in order to reduce the main side effects due to oncological treatments, potentially improve their therapeutic efficacy and enhance physical and emotional wellbeing during and after standard treatments. In the main developed countries, 40–50% of cancer patients use integrative therapies, either in United States and Europe, where the most widely used are herbal medicine, acupuncture, homeopathy and spiritual therapy (1,2). Considering the spread of the phenomenon, family doctors, oncologists and health care experts should be informed of this type of treatments, in order to help their patients in therapeutic protocols, integrating safe, rational and non-invasive approaches. This evidence-based integration of treatments may decrease the risk of patients rejecting proven conventional cancer therapies in favor of no scientific-based alternative methods as unique choice of cure. Furthermore, it is essential to have a primary understanding of herbal or nutrients properties, in order to avoid potential interactions with drugs. To date, no significant evidence is available yet that integrative therapies positively influence the prognosis of breast cancer (BC); however, some of the complementary approaches aimed at improving symptoms, adherence to mainstream cure and better results are backed up by sound scientific data. This paper summarizes the state of the art in clinical and scientific evidences about the main complementary treatments currently used in BC management.
Diet, nutrition, physical activity and risk of primary and recurrent breast cancer
It is widely known that diet and physical activity play a major role in preventing chronic and inflammatory diseases, including cancer (3). In this scenario, integrative and holistic approach, through a careful listening to patient’s needs and behaviors, must encompass a tailored counseling on lifestyles and encourage change in wrong habits, if needed. This implies first of all a thorough discussion of the most updated evidences about lifestyles’ role in tumor growth and progression, compliance to oncological protocols and primary and tertiary prevention of recurrences; patient’s information and education can also be achieved by indicating the best evidence-based references available on media and web. Secondly, patient must be motivated to change, even with a specific psychologic support, and goal achievements must be monitored by health care professionals. A recent and updated systematic review conducted by World Cancer Research Fund (WCRF) on “Diet, Nutrition, physical activity and Breast Cancer” (3) highlighted the correlation between body composition including BMI, waist-hip ratio and body fatness, physical activity, food and risk in at least 13 types of cancer. A meta-analysis based on 16 studies confirms the link between alcohol intake and BC risk, with a 5% increased risk per 10 g/day of ethanol (RR 1.05; 95% CI: 1.02–1.08) in pre-menopausal women and 9% per 10 g/day in post-menopausal (RR 1.09; 95% CI: 1.07–1.12) (3). Several researches focused on the role of non-starchy vegetables on BC risk show different kind of evidences in relation to estrogen receptor status; in 20 cohort studies including 993,446 women, followed for 11 to 20 years, an inverse association between total fruit and vegetable intake and risk of ER negative BC was noticed (RR 0.82; 95% CI: 0.74–0.90), but not for cancers overall or ER positive tumors (4). Mediterranean diet (MD) represents one of the healthiest model worldwide, recognized in 2010 as Intangible Cultural Heritage of Humanity by UNESCO, and therefore can play a key role in cancer management. PREDIMED trial on 4,282 women shows that MD including extra virgin olive oil (EVOO) (1 L/week) has a 62% relatively lower risk of malignant BC than those in the control arm diet (95% CI: 0.16–0.87) while participants in the MD supplemented with mixed nuts (30 g/d: 15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds) showed a nonsignificant risk reduction compared with women in the control group (HR, 0.62; 95% CI: 0.29–1.36) (5). Moreover, a diet with reduced intake of refined carbohydrates, saturated fats and increased consumption of whole-grain cereals, pulses and vegetables can positively affect levels of biomarkers related to cancer risk like insulin, sex hormones, insulin-like growth factor (IGF)-I binding proteins and IGF-1 in post-menopausal women (6-9) Among foods with specific antitumoral activities, so called functional foods or nutraceutics, ω-3 polyunsaturated fatty acids (PUFA) are important modulators of inflammation and known to be beneficial in the prevention of many chronic diseases that involve phlogistic processes, like cancer and cardiovascular diseases. In preclinical studies, ω-3 PUFA showed even an advantageous modulation of oncogenesis that may be useful in the adjuvant setting, but other studies are needed to confirm this hypothesis (10). Sulforaphane, a natural molecule of glucosinolates class, found in vegetables such as cabbage, broccoli and broccoli sprouts, has been proved to inhibit, both in vitro and in vivo, BC stem cell growth, suggesting a role in chemoprevention and adjuvant setting (11). Moreover, there are increasing findings that support the relationship between EVOO’s use and improvement of BC patient’s outcomes. EVOO is the most important dietary source of ω-9 fatty acids such as oleic acid, which seems to suppress Her-2/neu overexpression and interact with anti-Her-2/neu immunotherapy by enhancing the cell death of Her-2 positive BC cells (12). In addition, EVOO is rich in phenolic compounds that give the bitter and pungent taste to the oil; in vitro, the phenolic molecules show direct antioxidant property and are even able to enhance the effects of aromatase inhibitors (13). Very convincing evidences about body composition, including BMI and body fatness, and cancer risk and prognosis were found. A large analysis on 56 studies (n=80,404 cases) about BMI shows a dose-response increased risk of 12% per 5 kg/m2 for all breast cancer cases in post-menopause and mortality (RR 1.12; 95% CI: 1.09–1.15) but not in pre-menopausal BC (n=16,371 cases) with a 7% decreased risk per 5 kg/m2 (RR 0.93; 95% CI: 0.90–0.97). A recent review on body fatness in young adulthood (aged 18–30 years) based on 4,953 cases showed a 18% decreased risk per 5 kg/m2 (RR 0.82; 95% CI: 0.76–0.89) both for pre-menopausal BC and post-menopausal BC (RR 0.82; 95% CI: 0.76–0.88) (n=10,229 cases) (3). Various hypotheses were suggested to explain the biochemical mechanism that drives body fatness protection in young adulthood toward BC, including slower adolescent growth due to lower level of IGF-I recorded in fatter young women (3).
Beside dietary recommendations, physical activity (PA) is generally proposed both for prevention of primary cancers and recurrences. The benefits of PA derive from several mechanisms, including reduction of fatness overall and in specific critical anatomical regions, such as visceral and liver fat that impact endocrine and growth factors profiles. Moreover, exercise enhances insulin sensitivity, reduces fasting insulin and C-peptide levels (14) and has immunomodulatory effects that promote immune-surveillance and elimination of cancer cells (15). In a meta-analysis on 11,789 cases, PA was linked to a 13% decreased risk of BC between the highest and the lowest level of exercise (RR 0.87; 95% CI: 0.79–0.96) (3).
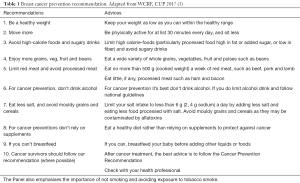
According to the most updated evidences collected so far, WCRF published several recommendations on diet, nutrition, physical activity and breast cancer, summarized in Table 1.
Diet and physical activity are also crucial during cancer treatments, in order to improve side effects management, enhance compliance to protocols and avoid malnutrition. Recently, the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Partnership for Action Against Cancer (EPAAC), released guidelines on nutrition in cancer patients (16). The aim of this document is to translate current evidences and expert opinions into recommendations for identification, prevention, and treatment of reversible elements of malnutrition in patients with cancer. In fact, cancer patients have catabolic alterations and systemic inflammation syndrome that negatively impact quality of life, physical function and treatment tolerance. All patients, particularly in advanced stage of disease, are strongly invited to follow the guidelines in order to avoid lean mass loss and must be screened to detect nutritional risks at an early stage, regularly evaluate nutritional intake, total energy expenditure and weight change. If not measured individually, nutritional intake must be assumed as ranging between 25 and 30 kcal/kg/day, with a protein intake above 1 g/kg/day and, if possible, up to 1.5 g/kg/day in patients with malnutrition risk. In weight-losing cancer patients with insulin resistance, is recommended to increase energy ratio coming from fat, in order to increase energy density of diet and reduce the glycemic load. Vitamins and minerals have to be supplied in amounts approximately equal to the RDA and discourage the use of high-dose micronutrients, in the absence of specific deficit. It is also suggested the use of supplementation with long-chain ω-3 PUFA or fish oil to stabilize or enhance appetite, food intake, lean body mass and body weight in patients with advanced cancer undergoing chemotherapy with risk of weight loss or malnutrition. Unlike other cancer patients, women diagnosed with BC often undergo weight gain due to hormonal imbalance and menopausal state induced by treatments, during which a specific diet aimed at weight management may play an important role. In fact, obesity and weight gain are related with poorer prognosis, as well as prevalent comorbid conditions, such as cardiovascular disease, metabolic syndrome and diabetes, poorer surgical outcomes in terms of recovery times and infection rates; in addition, weight influences lymphedema, fatigue, bone health and overall quality of life (17). A promising horizon of research in this field is caloric restriction. Preclinical studies show that caloric restriction has beneficial effects in growth hormone (18), IGF-1 (19) and insulin levels (20) and sensitivity (21), but also in blood glucose (22), nitrogen (23), free fatty acids and triglycerides (24). Moreover, preclinical data researches show that weight loss from caloric restriction and increased PA may modulate hormonal and inflammatory factors that influences neoplastic progression; however, exact mechanisms are not already know, and experiments in humans are limited (25). As recommended for cancer prevention, physical activity is equally fundamental during oncological treatments, and maintenance of PA in this setting is useful to improve muscle mass, physical function and metabolic pattern. In a recent study collecting prospective data on 959 breast cancer survivors from the Diet, Cancer, and Health cohort, in adjusted analyses, self-reported exercise above eight MET h/week, compared to lower levels of activity, was significantly associated with improved overall survival (HR, 0.68; CI: 0.47–0.99). A risk reduction of 44% was found in overall survival (HR, 0.56; CI: 0.33–0.95), comparing participation in exercise to control arm. Pre-diagnosis exercise, BMI, and receptor status of disease did not change significantly the results (26). A daily brisk walk is proven to improve also mood disorders, sleep disturbances and cancer-related fatigue, even during active oncologic treatments (27).
Another important issue related to lifestyles regards the microbiota, composed of symbiotic bacteria, yeast and other microorganisms that mainly colonize the gastro-intestinal tract and other epithelial barriers of the host, including skin, who can be well described as a human holobiont. The composition of the microbiota, in fact, develops from the birth to the third year of life, before maturation and is regulated by host genetics, colonization at the time of birth, type of birth delivery, incidence of diseases, exposure to antibiotics and eventually by individual’s lifestyle, including diet and physical activity (28). Gut homeostasis is also preserved by constant crosstalk between the gut microbiota, immune cells and the mucosal barrier (29). In adulthood the composition of microbiota remains relatively constant, although it can still be influenced by diet, PA, pathological status and treatments. The microbiota located in several epithelia, mainly in the gut, impacts on local and systemic metabolic pathways, phlogosis and adaptive immunity, which regulate oncogenesis, evolution and response to anticancer treatment. Recently, it has become evident that microbiota modulates the tumor microenvironment, response to anticancer therapy and susceptibility to toxic side effects (30,31). It is well documented that diets rich in fiber and vegetables are associated with gut microbial changes that, in turn, are associated with a health benefit. Even if only a relatively small number of randomized, clinically controlled dietary interventions targeting the gut microbiota have been reported in humans, and investigation on the gut microbiome is in an early phase, the findings so far support the view that specific dietary habits, used alone or combined with the administration of mixtures of microbial species (next-generation synbiotics) (32), may hold potential for enhancing public health. Thus, targeting the microbiota in cancer, a brand-new branch of oncology named Oncobiotics, is likely to become one of the next frontiers for personalized medicine (33).
Based on these data, an integrative approach to cancer patients must include diet and PA counseling in mainstream oncological treatments, performed by specialists as part of the multidisciplinary team for a Precision Medicine model of care.
Complementary therapies
So far, there is no persuasive evidence that complementary therapies positively impact the natural history of BC, recurrence rates or survival endpoints, because very few adequately powered RCTs have examined the effect of integrative therapies on these outcomes.
Nevertheless, selected integrative approaches aimed at improving symptoms and adherence to standard cure are showing very promising results or are already backed up by sound scientific data. Cancer patients are burdened by symptoms related to the disease itself or to treatments toxicity, particularly diarrhea and constipation, hot flashes, lymphedema, fatigue, insomnia, mucositis, nausea and vomiting, neuropathy, radiodermatitis, xerostomia, frequently associated with mood and sleeping difficulties that can deeply impair quality of life. A systematic review also highlights that cancer pain is found in 33% of patients after therapeutic treatment, in 59% of patients following anticancer therapy and in 64% of people with metastatic cancer (34).
In the following paragraphs, we try to briefly summarize the most convincing evidence-based data about complementary approaches used in BC management so far, and the most validated clinical indications for each kind of procedures.
Acupuncture represents today a safe, reliable and cost-effective way of curing patients, if performed properly by a specialized practitioner, based on a patient-centered, rather than disease-centered approach. After almost 2,500 years of development in Traditional Chinese Medicine, acupuncture has demonstrated to treat successfully a several kinds of disorders, even addressing some of the unmet needs in cancer patients, with minimal or absent adverse reactions (35). During the past three decades, a growing number of studies on acupuncture have been realized, to achieve the statistical significance of this approach in comparison with mainstream care, even if in most cases the studies are not homogeneous and have not been conducted in a rigorous scientific manner. The objective difficulty to obtain a real “placebo” (often defined “sham” acupuncture, that is needling at random sites) and standardized protocols in different subsets of patients and clinical settings has so far limited high quality controlled trials, based on sound research methodology. Nevertheless, if controlled trials demonstrating that real acupuncture is better than sham acupuncture provide evidence showing the effectiveness of this approach, on the other hand negative results, in which both the real and sham acupuncture revealed considerable therapeutic effects, can hardly be taken as evidence that acupuncture is not effective, but at least that additional studies are needed. The proficiency of acupuncturists also represents another difficulty in evaluating acupuncture practice because the therapeutic effect depends greatly on their skill in selecting and locating the acupoints and in manipulating the needles. However, if improvements due to acupuncture in chronic and long-lasting diseases like cancer are reported by patients, this approach should be viewed in a more favorable light, particularly for conditions, such as iatrogenic menopause in hormonal responsive tumors, for which conventional treatments are ineffective or not available, as well as in conditions where the patient is scared about drugs side effects. A systematic review published by the National Cancer Institute, focusing on randomized clinical trials, prospective consecutive case series and retrospective studies involving acupuncture in chemotherapy and radiotherapy-induced symptoms, showed that the highest efficacy of acupuncture in oncology is for treating chemotherapy-induced nausea and vomiting (36). There have been few studies regarding acupuncture and anxiety or depression in oncologic research; some studies conducted on non-oncological patients however suggest that acupuncture and acupression are effective in treating depression and anxiety, with very few side effects compared to standard care (37). Lu et al. (38) reviewed several pilot not controlled clinical trials which show improvement in patients with fatigue related to chemotherapy: in patients with persistent asthenia after antiblastic therapy, acupuncture’s use resulted in an improvement of 31.3%. In a RCT, comparing acupuncture with enhanced usual care, involving 302 breast cancer patients, fatigue at 6 weeks, measured with the Multidimensional Fatigue Inventory (MFI), significantly improved in intervention arm (P=0.001). Acupuncture reduced also anxiety and depression scores, and overall quality of life (39). In a recent survey, 424 BC survivors who had completed oncological treatments at least 12 months previously were randomized into three arms (relaxing acupressure, stimulating acupressure or usual care). After 10 weeks, both acupressure types significantly reduced persistent fatigue compared with usual care, but only relaxing acupressure had significant effects on sleep and quality of life (40). According to the Guidelines published by the Society for Integrative Oncology (SIO), the acupuncture treatment is recommended in cancer-related fatigue as a support to therapy, but further studies are needed (41). In a review in BC patients experiencing hot flashes, acupuncture can improve the vasomotor symptoms, with benefits lasting up to 6 months even though there is little difference between the true and sham acupuncture (38). A systematic review including six RCTs on women undergoing adjuvant estrogen-antagonist treatment, acupuncture showed favorable results regarding the frequency of hot flashes (P>0.001), but marked heterogeneity was observed in this model (42). In a randomized trial on 120 BC survivors, comparing electroacupuncture versus gabapentin, acupuncture was more effective than pills, with fewer adverse effects, for the treatment of hot flashes (43). In a pragmatic randomized trial on 190 cancer patients, acupuncture in association with enhanced self-care was a useful integrative intervention for hot flashes management and improving quality of life (44). In conclusion, in patients with BC who experience vasomotor symptoms related to menopausal state, who do not respond to medication, treatment with acupuncture can be considered and may provide additional and longer-term benefits without adverse effects. Regarding acupuncture’s effectiveness on pain, the majority of high quality studies refer to non-oncological pain. A systematic review for acute postoperative pain management argued that postoperative pain intensity was significantly decreased in the acupuncture group and the acupuncture treatment group was associated with a lower incidence of opioid-related side-effects (45). The efficacy of acupuncture on cancer-related pain is less certain, and a Cochrane review on this topic confirms that positive results should be viewed with caution due to methodological limitations, small sample sizes, poor reporting and inadequate analysis (46). Nevertheless, the majority of authors believe that the results are promising, particularly for patients with comorbidity or not responder to conventional analgesic therapy. The review by Lu et al. also suggests that acupuncture can be used for chronic neuropathic pain and for postoperative pain in BC patients (38). The 2017 SIO Guidelines confirms that acupuncture can be recommended as a integrative therapy for cancer patients, particularly when side effects of drugs, such as opioids, are clinically significant (grade C recommendation) (41). In evaluating acupuncture practice in Oncology, and particularly in BC treatment, not only effectiveness, but also other factors, including safety, costs, availability and the condition of local health services must be considered. In developing countries, where medical personnel and medicines are still lacking, a proper use of this simple and low-cost therapy could help a large number of cancer patients. On the other hand, in developed countries, where the health system is well reputable and updated technologies available, but still exist economic limitations, acupuncture may play a role in obtaining good results against a number of treatment related side effects, while not interfering with mainstream therapies and helping patients in a better and faster recovery of a relative wellbeing, during and after oncological treatments.
Over the last 20 years, mind-body therapies including Mindfulness, Qi gong, yoga, Thai-Chi and meditation, have received increasing awareness and consideration from the scientific community seeking to understand the safety and efficacy of these broadly used practices. Mind-body therapies offer many psychological and health functioning benefits, including reductions in disease symptoms, empowerment in coping and well-being (47). Increasing evidence suggests that stress-reduction techniques of meditation (mindfulness-based stress reduction—MBSR) improve anxiety, depression and overall quality of life (strength of evidence grade A in SIO guidelines) (41,48-50). Practicing these techniques seems to be enjoyable and successful in reducing psychological and physical distress and effective in addressing unmet needs of BC patients undergoing active treatments. Because they are offered in group settings, meditation interventions are more cost effective than traditional individual counseling or psychotherapy and can often achieve similar results, with very low dropout rates. In a recent Cochrane meta-analysis of 24 studies with a total of 2,166 participants, assessing the potential role of yoga on quality of life and symptoms derived from cancer in women diagnosed with BC, the authors conclude that moderate-quality evidence supports yoga as a integrative intervention for enhancing health-related quality of life and reducing fatigue and sleep disturbances, when compared with no therapy, as well as for reducing depression, anxiety and fatigue, when compared with psychosocial/educational interventions (51). In a randomized trial on 162 cancer patients, regression analysis indicated that women doing Qi gong (an ancient Chinese practice of meditation) significantly improved overall QOL (P<0.001), fatigue (P<0.001), mood disturbance (P=0.021) and inflammation (CRP) compared with usual care (52). In another study, multilevel analyses revealed that women during radiation therapy for breast cancer in the Qi gong group reported lower level of depression than in the control arm (P=0.05), less fatigue (P<0.01) and better overall QOL (P<0.05), with clinically significant findings (53). A meta-analysis including thirty-four studies (total 2,219 participants) revealed that after 7 to 16 weeks of mind-body intervention, including Qi gong, Thai-Chi and yoga, there was a modest effect on reduction of C-reactive protein (effect size 0.58) and a small but not statistically significant reduction of interleukin-6 (effect size 0.35), suggesting a potential role of mind-body therapies in inflammation and immune system (54).
Medicinal plants contain many different constituents, with variable pharmacological activities, including effective anticancer compounds, that may be used as a support to mainstream cancer therapies to improve efficacy and/or reduce toxicity from chemotherapy including side-effects management. Based on a systematic review of the literature, the SIO states that there is a lack of strong evidence supporting the use of ingested dietary supplements as supportive care in daily management of breast cancer treatment-related side effects. Though, the most validated clinical indications of phytotherapy in BC patients are chemo-induced nausea and vomiting management, radiotherapy-induced dermatitis, gastrointestinal disorders and mucositis. For example, the antiemetic effect of ginger root on nausea and vomiting induced by chemotherapy was confirmed in a randomized, prospective, cross-over and double-blind study with a control of nausea was achieved in 62% of patients on ginger, 58% with metoclopramide and 86% with ondansetron, with no adverse effects (55). Ginger supplementation should not be used in perioperative settings or in patients with bleeding disorders, due to a potential risk of increased bleeding (56). The use of mistletoe in cancer care is based on the premise that injections of specially prepared extracts of the plant during chemotherapy and radiation therapy can create a host response that is immune-stimulatory, preferentially cytotoxic to cancer cells, and protective of host cells (57). Preparations from European mistletoe are some of the most common internationally prescribed substances in outpatient clinics for cancer; clinical evidence on the use of mistletoe is based on trials in which it is co-administered with conventional treatments to improve overall quality of life (58) (strength of evidence grade C in SIO guidelines). Cannabis sativa has been studied for its use in pain as well as in nausea and vomiting related to cancer treatment; cannabinoids inhibit pain either via central CB1 receptors or by peripheral CB2 receptors, which have been implicated in the control of “inflammatory” pain. Lynch et al. (59) analyzed, in a systematic review, the use of cannabinoids in chronic non-cancer pain conditions including neuropathic pain, fibromyalgia, rheumatoid arthritis, and mixed chronic pain. Fifteen of 18 trials selected demonstrated a significant analgesic effect of cannabinoid in comparison to a placebo. Cannabinoids are particularly effective against chronic neuropathic pain and in multiple sclerosis pain, but have little or no effect in patients with acute pain (60). Moreover, there are many scientific evidences about the action of cannabis in the treatment of generic pain, while few of these are RCTs on cancer related pain. Interestingly, some studies show that cannabis may have synergistic interaction with opioids in the treatment of pain, which could contribute to reduce doses and exposure to these currently overused drugs (61-63). When talking about herbs, dietary supplements and nutraceutics, one of the major concerns of practitioners is to avoid unfavorable interactions between drugs and other compounds; that explains the paramount importance of asking what the patients take during treatments and knowing the potential synergistic or antagonistic effects (56).
In patients with cancer, the goal of massage is to promote relaxation, address muscle stiffness and pain, and resolve musculoskeletal complaints. Massage therapists should take precautions to avoid massaging open wounds, a blood clot in a vein, a tumor site or sensitive skin after radiation therapy (64). In addition, certain patients with multiple bone metastases may be at risk for fracture during deep massage. Foot reflexology has proven to be effective in cancer patients as complementary therapy in relieving pain and anxiety; in the same study, patients received significantly less opioid analgesics than the control arm (65). A longitudinal, randomized clinical trial involving 385 women with advanced-stage BC, in chemotherapy and/or hormonal therapy, revealed significant improvements in physical functioning for the reflexology arm of the study compared to the control arm (P=0.04); particularly, severity of dyspnea was reduced in the reflexology arm compared to the control arm (P<0.01) (66).
A Cochrane review found no serious side effects or interactions when homeopathy was used for the adverse effects of radiotherapy and chemotherapy, with preliminary data in support of topical calendula for prophylaxis of acute dermatitis during radiotherapy and Traumeel S in reducing severity and duration of chemotherapy-induced stomatitis (67,68). Furthermore, Calendula officinalis reduces the incidence of pain and dermatitis in women receiving radiation for breast cancer (69). Arnica montana has been successfully used as a homeopathic remedy in pain management and postoperative settings (pain, edema, and ecchymosis) (70). Colau et al. (71) evaluated the effectiveness of the homeopathic non-hormonal treatment BRN-01 in reducing hot flashes in menopausal women, in a multicenter research including 108 patients. The treatment seemed to have a significant effect on hot flashes, compared with placebo, suggesting a potential role with a safe profile for menopausal symptoms in patients who cannot take hormone replacement therapy or other recognized treatments. Further and better designed studies are still needed in this fields to draw definitive conclusions about usefulness of homeopathy in breast cancer management.
The music therapy interventions are described as either passive or active, depending on the level of engagement required by the individual. Passive music therapy is recommended to reduce anxiety during radiation therapy, chemotherapy sessions, and postsurgery (SIO guidelines grade B) based on results from five RCTs comparing music therapy interventions with standard care (72-76). The sample sizes of these studies ranged from 30 to 170 participants. A recent systematic review and meta-analysis assessed the effect of different expressive therapies, including passive and active music therapy, on improving anxiety, depression, and QOL in patients with breast cancer (77). In another recent meta-analysis, it was found a clinically and statistically significant mean difference (P<0.01) in the anxiety scores of patients who received music therapy compared with the control group (77). Passive music therapy has also been shown to reduce anxiety among patients undergoing mammographic screening, indicating that the recommendation may apply broadly to adult women in a clinical cancer setting (78). Passive music therapy is noninvasive, it does not require costly, technologically advanced equipment and can be implemented in a variety of locations.
Conclusions
Integrative oncology combines lifestyle counseling, evidence-based complementary therapies with mainstream care, trying to improve physical, psychological and spiritual well-being of the patient, taking into consideration the individual’s values and priorities, improving woman’s comprehension of therapeutic choices, enhancing her active participation to the protocols. Awareness of the main integrative therapies available should be a core competence for any cancer care provider and should be applied in decision making for patients with cancer who require supportive care (79). Lots of resources are spent each year worldwide on complementary health therapies with unknown benefits or already proven to be ineffective; high quality clinical research in this area could save useless efforts and redirect patients to more appropriate treatments, with better safety profiles and known benefits. In a modern concept of Precision Medicine, such a patient-centered approach should be implemented to address unmet needs and integrated into standard cancer care to improve patient’s outcomes and quality of life.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Gianluca Franceschini, Alejandro Martín Sánchez, Riccardo Masetti) for the series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.02.06). The series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol 2005;16:655-63. [Crossref] [PubMed]
- John GM, Hershman DL, Falci L. Complementary and alternative medicine use among US cancer survivors. J Cancer Surviv 2016;10:850-64. [Crossref] [PubMed]
- World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: diet, nutrition, physical activity and breast cancer. 2017. Available online: http://www.aicr.org/continuous-update-project/reports/breast-cancer-report-2017.pdf
- Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst 2013;105:219-36. [Crossref] [PubMed]
- Toledo E, Salas-Salvadó J, Donat-Vargas C, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med 2015;175:1752-60. [Crossref] [PubMed]
- Berrino F, Bellati C, Secreto G, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev 2001;10:25-33. [PubMed]
- Berrino F, Pasanisi P, Bellati C, et al. Serum testosterone levels and breast cancer recurrence. Int J Cancer 2005;113:499-502. [Crossref] [PubMed]
- Kaaks R, Bellati C, Venturelli E, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr 2003;57:1079-88. [Crossref] [PubMed]
- Pasanisi P, Berrino F, De Petris M, et al. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer 2006;119:236-8. [Crossref] [PubMed]
- Zivkovic AM, Telis N, German JB, et al. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric (Berkeley) 2011;65:106-11. [Crossref] [PubMed]
- Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 2010;16:2580-90. [Crossref] [PubMed]
- Menendez JA, Vellon L, Colomer R, et al. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin™) in breast cancer cells with Her-2/neu oncogene amplification. Ann Oncol 2005;16:359-71. [Crossref] [PubMed]
- Ismail AM, In LL, Tasyriq M, et al. Extra virgin olive oil potentiates the effects of aromatase inhibitors via glutathione depletion in estrogen receptor-positive human breast cancer (MCF-7) cells. Food Chem Toxicol 2013;62:817-24. [Crossref] [PubMed]
- McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008;8:205-11. [Crossref] [PubMed]
- Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer 2010;46:2593-2604. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer 2012;118:2277-87. [Crossref] [PubMed]
- Shimokawa I, Higami Y, Tsuchiya T, et al. Life span extension by reduction of the growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. FASEB J 2003;17:1108-9. [Crossref] [PubMed]
- Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 2010;107:7467-72. [Crossref] [PubMed]
- Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One 2009;4:e4567 [Crossref] [PubMed]
- Escrivá F, Gavete ML, Fermin Y, et al. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol 2007;194:131-41. [Crossref] [PubMed]
- Al-Regaiey KA, Masternak MM, Bonkowski MS, et al. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci 2007;62:18-26. [Crossref] [PubMed]
- Felgines C, Savanovitch C, Farges MC, et al. Protein metabolism in rats during long-term dietary restriction: influence of aging. JPEN J Parenter Enteral Nutr 1999;23:32-7. [Crossref] [PubMed]
- Filaire E, DeGoutte F, Jouanel P, et al. Biological alterations after food restriction and training in rats. J Exerc Physiol Online 2004;7:37-44.
- Chlebowski Rowan T, Aiello Erin, et al. Weight loss in breast cancer patient management. J Clin Oncol 2002;20:1128-43. [Crossref] [PubMed]
- Ammitzbøll G, Søgaard K, Karlsen RV, et al. Physical activity and survival in breast cancer. Eur J Cancer 2016;66:67-74. [Crossref] [PubMed]
- Husebø AM, Dyrstad SM, Mjaaland I, et al. Effects of scheduled exercise on cancer-related fatigue in women with early breast cancer. ScientificWorldJournal 2014;2014:271828 [PubMed]
- Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369-79. [Crossref] [PubMed]
- Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 2008;4:447-57. [Crossref] [PubMed]
- Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967-70. [Crossref] [PubMed]
- Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015;350:1084-9. [Crossref] [PubMed]
- Neef A, Sanz Y. Future for probiotic science in functional food and dietary supplement development. Curr Opin Clin Nutr Metab Care 2013;16:679-87. [Crossref] [PubMed]
- Thomas RM, Jobin C. The microbiome and cancer: is the ‘oncobiome’mirage real? Trends Cancer 2015;1:24-35. [Crossref] [PubMed]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-49. [Crossref] [PubMed]
- Sagar SM. Acupuncture as an evidence-based option for symptom control in cancer patients. Curr Treat Options Oncol 2008;9:117-26. [Crossref] [PubMed]
- Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol 2013;31:952-60. [Crossref] [PubMed]
- Deng GE, Frenkel M, Cohen L, et al. Evidence-based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J Soc Integr Oncol 2009;7:85-120. [PubMed]
- Lu W, Dean-Clower E, Doherty-Gilman A, et al. The value of acupuncture in cancer care. Hematol Oncol Clin North Am 2008;22:631-48. viii. [Crossref] [PubMed]
- Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Supportive Care in Cancer 2013;21:1735-41. [Crossref] [PubMed]
- Zick SM, Sen A, Wyatt GK, et al. Investigation of 2 types of self-administered acupressure for persistent cancer-related fatigue in breast cancer survivors: a randomized clinical trial. JAMA Oncol 2016;2:1470-6. [Crossref] [PubMed]
- Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 2017;67:194-232. [Crossref] [PubMed]
- Sun Y, Gan TJ, Dubose JW, et al. Acupuncture and related techniques for postoperative pain: a systematic review of randomized controlled trials. Br J Anaesth 2008;101:151-60. [Crossref] [PubMed]
- Mao JJ, Bowman M, Xie S. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J Clin Oncol 2015;33:3615-20. [Crossref] [PubMed]
- Lesi G, Razzini G, Musti MA. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: a prospective multicenter randomized controlled trial (AcCliMaT). J Clin Oncol 2016;34:1795-802. [Crossref] [PubMed]
- Alimi D, Rubino C, Pichard-Léandri E, et al. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol 2003;21:4120-6. [Crossref] [PubMed]
- Paley CA, Johnson MI, Tashani OA, et al. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev 2011;CD007753 [PubMed]
- Schure MB, Christopher J, Christopher S. Mind–body medicine and the art of self-care: teaching mindfulness to counseling students through yoga, meditation, and qigong. J Counseling & Development 2008;86:47-56. [Crossref]
- Jacobsen PB, Phillips KM, Jim HS, et al. Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psychooncology 2013;22:1229-35. [Crossref] [PubMed]
- Aguado Loi CX, Taylor TR, McMillan S, et al. Use and helpfulness of selfadministered stress management therapy in patients undergoing cancer chemotherapy in community clinical settings. J Psychosoc Oncol 2012;30:57-80. [Crossref] [PubMed]
- Phillips KM, Antoni MH, Lechner SC, et al. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosom Med 2008;70:1044-9. [Crossref] [PubMed]
- Cramer H, Lauche R, Klose P, et al. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev 2017;1:CD010802 [PubMed]
- Oh B, Butow PN, Clarke SJ, et al. Impact of medical qigong on quality of life, fatigue, symptoms, mood and inflammation in cancer patient: a randomized controlled trial. Ann Oncol 2010;21:608-14. [Crossref] [PubMed]
- Chen Z, Meng Z, Milbury K, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer. Cancer 2013;119:1690-8. [Crossref] [PubMed]
- Morgan N, Irwin MR, Chung M, et al. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One 2014;9:e100903 [Crossref] [PubMed]
- Sontakke S, Thawani V, Naik MS. Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: a randomized, cross-over, double blind study. Indian J Pharmacology 2011;35:32-6.
- Memorial Sloan Kettering Cancer Center. Integrative Medicine: About Herbs, Botanicals and Other Products. Available online: https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs
- Marvibaigi M, Supriyanto E, Amini N, et al. Preclinical and clinical effects of mistletoe against breast cancer. Biomed Res Int 2014;2014:785479 [PubMed]
- Semiglazov VF, Stepula VV, Dudov A. Quality of life is improved in breast cancer patients by standardised mistletoe extract PS76A2 during chemotherapy and follow-up: a randomised, placebo-controlled, doubleblind, multicentre clinical trial. Anticancer Res 2006;26:1519-29. [PubMed]
- Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol 2011;72:735-44. [Crossref] [PubMed]
- Grotenhermen F, Müller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 2012;109:495-501. [PubMed]
- Cichewicz DL, McCarthy EA. Antinociceptive synergy between Δ9-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther 2003;304:1010-5. [Crossref] [PubMed]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci 2004;74:1317-24. [Crossref] [PubMed]
- Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol 2006;530:54-8. [Crossref] [PubMed]
- Gecsedi RA. Massage therapy for patients with cancer. Clin J Oncol Nurs 2002;6:52-4. [Crossref] [PubMed]
- Tsay SL, Chen HL, Chen SC, et al. Effects of reflexotherapy on acute postoperative pain and anxiety among patients with digestive cancer. Cancer Nurs 2008;31:109-15. [Crossref] [PubMed]
- Wyatt G, Sikorskii A, Rahbar MH, et al. Health-related quality-of-life outcomes: a reflexology trial with patients with advanced-stage breast cancer. Oncol Nurs Forum 2012;39:568-77. [Crossref] [PubMed]
- Kassab S, Cummings M, Berkovitz S, et al. Homeopathic medicines for adverse effects of cancer treatments. Cochrane Database Syst Rev 2009;CD004845 [PubMed]
- Oberbaum M, Yaniv I, Ben-Gal Y. Homeopathic Medication TRAUMEEL S reduces chemotherapy-induced stomatitis in children. Cancer 2001;92:684-90. [Crossref] [PubMed]
- Pommier P, Gomez F, Sunyach MP, et al. Phase III randomized trial of Calendula officinalis compared with Trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol 2004;22:1447-53. [Crossref] [PubMed]
- Iannitti T, Morales-Medina JC, Bellavite P, et al. Effectiveness and Safety of Arnica montana in PostSurgical Setting, Pain and Inflammation. Am J Ther 2016;23:e184-97. [Crossref] [PubMed]
- Colau JC, Vincent S, Marijnen P, et al. Efficacy of a non-hormonal treatment, BRN-01, on menopausal hot flashes: a multicenter, randomized, double-blind, placebo-controlled trial. Drugs R D 2012;12:107-19. [Crossref] [PubMed]
- Binns-Turner PG, Wilson LL, Pryor ER, et al. Perioperative music and its effects on anxiety, hemodynamics, and pain in women undergoing mastectomy. AANA J 2011;79:S21-7. [PubMed]
- Bulfone T, Quattrin R, Zanotti R, et al. Effectiveness of music therapy for anxiety reduction in women with breast cancer in chemotherapy treatment. Holist Nurs Pract 2009;23:238-42. [Crossref] [PubMed]
- Hanser SB, Bauer-Wu S, Kubicek L, et al. Effects of a music therapy intervention on quality of life and distress in women with metastatic breast cancer. J Soc Integr Oncol 2006;4:116-24. [Crossref] [PubMed]
- Li XM, Zhou KN, Yan H, et al. Effects of music therapy on anxiety of patients with breast cancer after radical mastectomy: a randomized clinical trial. J Adv Nurs 2012;68:1145-55. [Crossref] [PubMed]
- Zhou K, Li X, Li J, et al. A clinical randomized controlled trial of music therapy and progressive muscle relaxation training in female breast cancer patients after radical mastectomy: results on depression, anxiety and length of hospital stay. Eur J Oncol Nurs 2015;19:54-9. [Crossref] [PubMed]
- Boehm K, Cramer H, Staroszynski T, et al. Arts therapies for anxiety, depression, and quality of life in breast cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2014;2014:103297 [PubMed]
- Zavotsky KE, Banavage A, James P, et al. The effects of music on pain and anxiety during screening mammography. Clin J Oncol Nurs 2014;18:E45-9. [Crossref] [PubMed]
- Magno S, Filippone A, Scaldaferri A, et al. Integrative approaches in breast cancer patients: A mini review. Integr Cancer Sci Therap 2016;3:460-4. [Crossref]