
Non-small cell lung carcinoma: understanding cancer microenvironment to drive immunotherapy and patients’ selection
Cancer microenvironment, immunotherapy and patients’ selection
Lung cancer is still the first cause of cancer-related death worldwide with non-small cell lung carcinoma (NSCLC) determining approximately 85% of all cases (1). Almost half of lung cancers present with metastases at the diagnosis. Several clinical options have been used, but prognosis remains poor especially in the advanced stages with about 5% of survivors after 5 years (2). Platinum-based chemotherapy remains the standard of care in the first line of treatment, although with a low rate of response ranging between 15–30% (3). In this scenario in the last few years, identification and targeting of programmed cell death-1 (PD-1) and one of its ligands, programmed cell death-ligand 1 (PD-L1), have shown extraordinary results in several cancer types. Consequently, nivolumab and pembrolizumab, two anti-PD-1 agents, has been approved by Food and Drug Administration (FDA) and European Medicines Agency (EMA) for first- or second-line of therapy in NSCLC.
In a recent issue on Clinical Cancer Research, Mazzaschi et al. (4) report the results of their study, where they evaluated if different tissue immune microenvironments were able to predict survival and response to immune checkpoint inhibitors (ICIs) in NSCLC. The cohort comprised 100 patients undergoing lung resections with curative intent and 26 patients with advanced disease treated with nivolumab in second or third line therapy. Hence, tumor infiltrating lymphocytes (TILs) and PD-1/PD-L1 expression and quantification were assessed. The authors found that NSCLC resected patients with high CD8 lymphocytes lacking PD-1 inhibitor receptor had a longer overall survival and PD-1-to-CD8 ratio was a prognostic factor on both univariate and multivariate analysis. Accordingly, they indicated CD8 lymphocytes lacking PD-1 inhibitor receptor as potential new factor of positive prognosis and survival. The other important findings were that, among patients treated with nivolumab, those with clinical benefit had low PD-1-to-CD8 ratio compared to non-responders and a significant prolonged progression-free survival (median PFS =12.96 vs. 1.84, respectively). Moreover, the incidence and phenotype of TILs differed in squamous cell carcinoma (SCC) versus adenocarcinoma (ADC), in which EGFR and KRAS mutations conditioned a different frequency and tissue localization of lymphocytes. Therefore, some reflections have to be reported, in particular regarding PD-1 as a potential predictive biomarker and selection criterion. In fact, both staining intensity and quantification of positive cells by immunohistochemistry have not still demonstrated consistent results (5). Other issues on PD-1 evaluation include tissue preparation, processing variability, intra-tumor heterogeneity, staining of tumor versus immune cells. Moreover, prior treatments, i.e., radiation or chemotherapy, may also affect PD-1 expression (6). Consequently, it is not surprising that some studies, performed in different types of cancers, have shown clinical responses also in patients with low or no expression of PD-L1 (7,8).
TILs are a fundamental component of tumor microenvironment and play a significant role in the tumor biology and mediate response to ICI. It is known that a tumor with inflammatory milieu seems better to predict response to ICI. Indeed, such a tumor is characterized by CD8 T-cell infiltration, which leads to the cytotoxic effect typical of T-cell response (9). As a countermeasure tumor cells secrete cytokines, i.e., IL-10, which attract and promote regulatory T-cell (Treg) proliferation and suppress killing CD8 T cell-mediated (10,11). The results of Mazzaschi et al. (4) open an interesting point of view on the inhibition mechanism by ICI. As mentioned above, percentage of patients with good response notwithstanding low PD-L1 expression ranges between 20–25%. This aspect suggests a possible alternative pathway other than PD-1/PD-L1 involved in blocking the inhibition signaling between tumor cells and immune system (12), as appears evident in the Mazzaschi’s cohort where patients with CD8 lymphocytes lacking PD-1 had a better survival compared to others. Additionally, some authors argue that the function of CD8 T-cells having low or intermediate levels of PD-1 expression is enhanced by selectively PD-1 blockade, whereas CD8 T-cells expressing the highest levels of PD-1 are actually addressed to cell death (13). Therefore, a recent study evaluating the levels of PD-1 on TILs showed no significant correlation between PD-L1 expression in solid tumors and prediction of response to nivolumab (14). As stated by Mazzaschi et al. (4), also the role of other immune cells should be deepened. Myeloid derived suppressor cells (MDSC) are an assortment of non-macrophage cells; the name derived from their myeloid origin and their aptitude to depress T-cell functions. Two types of MDSC have been identified: monocytic MDSC (M-MDSC) and granulocyte polymorphonuclear MDSC (PMN-MDSC). The former in the tumor microenvironment differentiates into immune-suppressive tumor-associated macrophage, the most representative not malignant cells, which favorite tumor progression and inactive innate and adaptive immune response through several mechanisms, as well as PMN-MDSC, a group of cells sharing some characteristics with neutrophils (15). Moreover, in these type of cells it has been demonstrated an over-expression of PD-L1 via hypoxia inducible factor-1α (HIF-1α). Accordingly, combining HIF-1α inhibitors along with PD-L1/PD-1 blockade may be a new tool to enhance immune system in cancer patients (16). However, given the confusing PD-1/PD-L1 results, in our opinion, immunohistochemistry staining may not be considered the best biomarker for therapy with ICI. Other emerging biomarkers that might help predict response to ICI are based on serum or blood-related measurements. In fact, Weber et al. (17) have identified a group of serum proteins, namely acute phase complement and wound healing molecules, expressed in melanoma patients receiving anti-PD-1 antibodies characterized by poor prognosis. Nevertheless, the complexity of interactions between tumor cells, microenvironment and circulating proteins is still far from being completely elucidated and further clinical trials are warranted (Figure 1).
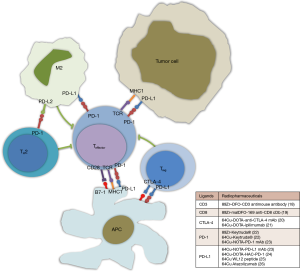
Non-invasive characterization of tumor microenvironment
The abovementioned observations have lead us to search innovative strategies to find out possible new biomarkers of response. In Mazzaschi’s study (4), all patients before lung surgery have performed 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), an essential imaging modality in diagnosis and staging of lung cancer (27). It might be of particular interest to analyze metabolic PET parameters as possible predictors of survival and response to immunotherapy. FDG uptake, mediated by overexpression of glucose transporter 1 (GLUT1), reflects biological features of tumors, such as proliferation, histologic type, and hypoxia. Additionally, FDG accumulates in tumor-related activated immune cells other than cancer cells (28). Recently, a correlation between metabolic information of FDG and tissue expression of immune markers in patients with NSCLC before surgery was reported (29). In particular, a significant association was found between SUVmax and SUVmean with the expression of CD8-TILs and PD-1-TILs. Moreover, another recent paper by Takada et al. (30) showed a statistically significant association between SUVmax and PD-L1 tumor expression in surgically resected lung cancer, suggesting PD-L1 expression as a malignant feature of the tumor. Indeed, as illustrated by Chang et al. (31) in a mouse model, high glucose consumption by tumors metabolically restricts T-cells, thereby allowing tumor progression. However, standardized methods to define the cut-off values for PET parameters has not yet established and larger sample size studies are needed, as well as tailored response evaluation criteria remain a challenge.
Immune-PET, a molecular imaging based on monoclonal antibodies or antibody fragments labelled with radioactive elements, represents a novel technique to determine in vivo the expression of cell surface markers of disease. The immune-PET term also includes the use of molecules not implied in targeting checkpoint inhibitors, such as CD3 or CD8 expression on T-cell surface (Figure 1). This innovative non-invasive approach may have a great impact on clinical activity, supporting oncologists in identification, stratification and early evaluation of response to ICI, thus allowing for a better understanding of the mechanisms of response to immunotherapy. On the other hand, improving the selection of optimal patients could address the economical aspect of immunotherapies, due to the high costs related to the prolonged treatment. However, most of these new immune-PET tracers have been investigated only in pre-clinical settings, so that several questions have to be resolved before application in clinical routine (18-26,32).
In conclusion, in order to better select patients that could benefit from these new drugs, the identification of potential predictive biomarkers represents a continuous challenge in oncology. In our opinion, a combination of cancer biomarkers should be considered. It is possible that different TILs features may have a role in the next future for driving the treatment of NSCLC, but further larger randomized studies are necessary.
Acknowledgments
Funding: The Italian Association for Research on Cancer (AIRC–Associazione Italiana per la Ricerca sul Cancro) is acknowledged for the support on this research.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Jun Zhou (Department of Nuclear Medicine, Zhongshan Hospital, Fudan University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.10). A Castello is supported with a fellowship related to the grant No. 18923 provided by AIRC to E Lopci.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Gulley JL, Spigel D, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015;33:abstr 8034.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-smallcell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 Expression in Cytotoxic CD8(+) Tumor-Infiltrating Lymphocytes Confers an Immune-Privileged Tissue Microenvironment in NSCLC with a Prognostic and Predictive Value. Clin Cancer Res 2018;24:407-19. [Crossref] [PubMed]
- Matos LL, Trufelli DC, de Matos MG, et al. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights 2010;5:9-20. [Crossref] [PubMed]
- Rossi S, Toschi L, Castello A, et al. Clinical characteristics of patient selection and imaging predictors of outcome in solid tumors treated with checkpoint-inhibitors. Eur J Nucl Med Mol Imaging 2017;44:2310-25. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Ghiotto M, Gauthier L, Serriari N, et al. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol 2010;22:651-60. [Crossref] [PubMed]
- Neurath MF, Finotto S. The emerging role of T cell cytokines in non-small cell lung cancer. Cytokine Growth Factor Rev 2012;23:315-22. [Crossref] [PubMed]
- Zhang CY, Qi Y, Li XN, et al. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomed Pharmacother 2015;69:242-8. [Crossref] [PubMed]
- Teng MW, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015;75:2139-45. [Crossref] [PubMed]
- Blackburn SD, Shin H, Freeman GJ, et al. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A 2008;105:15016-21. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol 2018;200:422-31. [Crossref] [PubMed]
- Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014;211:781-90. [Crossref] [PubMed]
- Weber JS, Sznol M, Sullivan RJ, et al. A Serum Protein Signature Associated with Outcome after Anti-PD-1 Therapy in Metastatic Melanoma. Cancer Immunol Res 2018;6:79-86. [Crossref] [PubMed]
- Larimer BM, Wehrenberg-Klee E, Caraballo A, et al. Quantitative CD3 PET imaging predicts tumor growth response to anti-CTLA-4 therapy. J Nucl Med 2016;57:1607-11. [Crossref] [PubMed]
- Tavaré R, Escuin-Ordinas H, Mok S, et al. An effective Immuno- PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res 2016;76:73-82. [Crossref] [PubMed]
- Higashikawa K, Yagi K, Watanabe K, et al. 64Cu-DOTA-antiCTLA-4 mAb enabled PET visualization of CTLA-4 on the Tcell infiltrating tumor tissues. PLoS One 2014;9:e109866 [Crossref] [PubMed]
- Ehlerding EB, England CG, Majewski RL, et al. ImmunoPET imaging of CTLA-4 expression in mouse models of non-small cell lung cancer. Mol Pharm 2017;14:1782-9. [Crossref] [PubMed]
- Natarajan A, Mayer AT, Reeves RE, et al. Development of Novel ImmunoPET Tracers to Image Human PD-1 Checkpoint Expression on Tumor-Infiltrating Lymphocytes in a Humanized Mouse Model. Mol Imaging Biol 2017;19:903-14. [Crossref] [PubMed]
- Hettich M, Braun F, Bartholoma MD, et al. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics 2016;6:1629-40. [Crossref] [PubMed]
- Maute RL, Gordon SR, Mayer AT, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci U S A 2015;112:E6506-14. [Crossref] [PubMed]
- Chatterjee S, Lesniak WG, Miller MS, et al. Rapid PD-L1 detection in tumors with PET using a highly specific peptide. Biochem Biophys Res Commun 2017;483:258-63. [Crossref] [PubMed]
- Lesniak WG, Chatterjee S, Gabrielson M, et al. PD-L1 Detection in Tumors Using [(64)Cu]Atezolizumab with PET. Bioconjug Chem 2016;27:2103-10. [Crossref] [PubMed]
- Takeuchi S, Khiewvan B, Fox PS, et al. Impact of initial PET/CT staging in terms of clinical stage, management plan, and prognosis in 592 patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:906-14. [Crossref] [PubMed]
- Kaira K, Serizawa M, Koh Y, et al. Biological significance of 18F-FDG uptake on PET in patients with non-small-cell lung cancer. Lung Cancer 2014;83:197-204. [Crossref] [PubMed]
- Lopci E, Toschi L, Grizzi F, et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patienst with non-small cell lung cancer (NSCLC) who are candidate for upfront surgery. Eur J Nucl Med Mol Imaging 2016;43:1954-61. [Crossref] [PubMed]
- Takada K, Toyokawa G, Okamoto T, et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med 2017;6:2552-61. [Crossref] [PubMed]
- Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015;162:1229-41. [Crossref] [PubMed]
- Natarajan A, Mayer AT, Xu L, et al. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjug Chem 2015;26:2062-9. [Crossref] [PubMed]


