
Expression and clinical significance of negative costimulatory molecules B7-H1, B7-H3 and B7-H4 in the process of colorectal cancer’s evolution
Introduction
Colorectal cancer (CRC) is a common cancer in humans and ranks fourth in the world for morbidity and mortality (1). In China’s economically developed areas, it is closer to that found in Western countries, and these areas are ranked second in malignant tumors. Therefore, exploring a mechanism to prevent and reverse the occurrence of CRC has become a major concern in basic research and clinical research in our country.
Now that the occurrence of CRC showed normal from the organization, through polyps, adenomas, high-grade tumor lesions such as the slow progression of cancer to the long process, involves a number of molecular pathways (2). Costimulatory signals and costimulatory molecules were first proposed and confirmed by Brestcher and Cohn in 1970 based on T cell activation dual signal theory (3). The regulatory of negative costimulatory molecules, their interactions and signaling play an extremely essential part in very complex immune response. Of the costimulatory molecules, the B7 family is the most important (4). These costimulatory signaling molecules provide positive signals for T cell growth, differentiation, and cytokine production. They also limit, weaken, or terminate T cell immune responses by providing negative signals and mediating immune escape by a variety of mechanisms that allow tumor cells to escape an immune attack (5). The current study suggests that B7-H1, B7-H3, B7-H4 which are negative costimulatory molecules belong to the B7 family. They are all expressed and negatively regulate the immune response by restraining T cell activation and proliferation with tumor cells, which takes an extremely important part in the tumor cell immune escape mechanism (6-8). The expression of these three negative costimulatory molecules proteins can be detected in many human tumor tissues (9). Its proteins are widely expressed in many tumor tissues, including kidney cancer, gastric carcinoma, prostate carcinoma, renal carcinoma, and neuroblastoma, and they are also found in tumor blood vessels (10-12).
The present study suggests that the interaction of PD-L1 with its receptor PD-1, which is expressed on T cells, leads to tumor antigen-specific T cell apoptosis. This is the main mechanism of B7-H1-mediated tumor immune escape (13). Negative correlation between B7-H1 expression and epithelial cell CD8+ T cell infiltration in ovarian cancer (14). This indicates that B7-H1 is expressed directly in tumor cells, and this has a direct inhibitory effect on the CD8+ T cells of tumor cells (15-17). High expression of B7-H4 can protect epidermal cells from anoikis and promote the malignant transformation of epithelial cells (18,19). Thus, it has an important position in the mechanism of immune escape in malignant tumors.
The development of CRC is a slow and lengthy process. However, B7 family are negative costimulatory molecules. They are found in different stages of tumor microenvironment formation and promote tumorigenesis and progression. It is unclear how they exert their respective immunological negative regulatory effects. Therefore, the study of B7 family molecules in the development of CRC could significantly benefit the early detection and improve the therapeutic effect of colorectal carcinoma.
Methods
Patients and tissue samples
Approved by the Ethical Review Committee of the Affiliated Hospital of Jiangnan University, 98 cases of CRC undergoing surgical treatment and pathological diagnosis at the Affiliated Hospital of Jiangnan University from 2006 to 2008 were collected. Into the group of cases, the tissue wax block is well preserved. For each case, all of the original hematoxylin and eosin-stained sections was reviewed by the researchers. The analysis of the clinical and pathological factors included the patient sex, age, tumor size, primary tumor (pT), histological grade, lymph node metastasis (pN), pathological stage, vascular invasion, nerve infiltration, and lymphatic infiltration. The average follow-up duration was 50.0 months, with a range of 0.8–104.0 months.
Immunohistochemistry (IHC) assay
IHC assay was performed according to previously described methods using antibodies for B7-H1 (ab205921, Abcam, Hongkong, China), B7-H3 (ab227679, Abcam, Hongkong, China), and B7-H4 (ab209242, Abcam, Hongkong, China). The analysis was the double-blind method was used to investigate the levels of these three negative costimulatory molecules in the B7 family in the HCC tissues. The proportion of tumor cells was scored using the following rules: zero points for no tumor cell staining; one point for 1–25% tumor cell coloring; two points for 26–50% tumor cell coloring; three points for 51–75% tumor cell coloring; four points for >75% tumor cell coloring. Protein expression intensity was scored as follows: zero points for no staining, one point for weak staining (light yellow), two points for moderate staining (yellowish brown), or three points for strong staining (brown). The staining index was calculated as the sum of the staining intensity and the proportion of tumor cell scores ratio (0, 1, 2, 3, 4, 6, 8, 9, or 12 points). Cut-off values for B7 family molecules expression were selected based on a measurement of heterogeneity using the log-rank test with respect to the overall survival. If the patients were classified as having high expression of B7-H1, the IHC scores were >6.25. Using the same method, if the patients were classified as having high expression of B7-H3, the IHC scores were >2.75, and high expression of B7-H4 was determined by IHC scores >5.75.
Flow cytometry analysis
To determine TIGIT expression in CD3+ T cells, mononuclear cells of 3 fresh tumor tissues and 3 adjacent normal tissues were incubated with fluorescein isothiocyanate-conjugated antihuman CD3, allophycocyanin-conjugated antihuman CD3, and either phycoerythrin-conjugated anti-B7-H1 (ab205921, Abcam, Hongkong, China), B7-H3 (ab227679, Abcam, Hongkong, China), or B7-H4 (ab209242, Abcam, Hongkong, China) antibody for 30 min at room temperature. After two washes with phosphate buffered saline (PBS), the PBMCs were analyzed using a flow cytometer (FACSCanto II; BD Biosciences, San Jose, CA, USA).
Statistical analysis
The most appropriate cutoff value for B7-H1, B7-H3, and B7-H4 scores were obtained by generating receiver operating characteristics (ROC) curves. The association between B7-H1, B7-H3 and B7-H4 expression and clinicopathological parameters was assessed by Pearson chi square test or Fisher exact test. Kaplan-Meier method was used to determine the possibility of overall survival, and logarithmic rank test was used to analyze the data. ROC curve (AUC) and evaluated multivariate analysis can predict the prognosis of CRC patients. The SPSS software package (version 20, IBM, Chicago, IL, USA) was used for statistical analysis, and P value <0.05 was deemed to significant.
Results
Expression of B7 family molecules in different stages of CRC progression
Immunohistochemical analysis showed that B7 family molecules (Figure 1) were expressed in the cytoplasm, cell membrane, as well as nucleus of tumor cells. There was nearly no expression of its found in normal tissues. However, the expression of B7-H1 and B7-H3 was significantly higher in polyps, adenomas, as well as high grade tumors than in benign tissues.
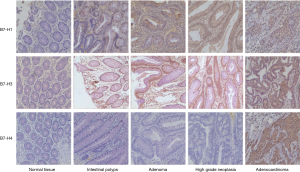
Expression rates of B7 family molecules in different stages of CRC progression
The B7-H1 expression rates in polyps, adenomas, and high-grade neoplasm tissues were 36.7%, 33.3%, and 44.0%, respectively. The B7-H3 expression rates in polyps, adenomas, and high-grade neoplasm tissues were 30.0%, 33.3%, and 48.0%, respectively. Among 98 CRC patients, 45.9% highly expressed B7-H1, 45.9% highly expressed B7-H3, and 57.1% highly expressed B7-H4 (Table 1).

Full table
Expression patterns of B7 family in different stages of colorectal carcinoma progression
The expression of the B7 family negative co-stimulatory molecules in different stages of CRC progression had different subcellular localization patterns: cell membrane expression, cytoplasmic expression, and nuclear expression (Figure 2A,B,C,D). The B7-H1, B7-H3 molecules were primarily expressed in the nucleus during the polyp stage. B7-H3 molecules were also expressed in the cytoplasm and the membrane, but B7-H4 molecules were not. The B7-H1, B7-H3 molecules were primarily expressed in the nucleus during the adenoma stage, and they were also expressed in infiltrating lymphocytes. The B7-H4 molecules were not expressed in infiltrating lymphocytes. B7-H1 and B7-H3 were primarily expressed in the cytoplasm and membrane during the high-grade neoplasia stage. B7 family were primarily expressed in the cytoplasm and the cell membrane during the tumor stage; they also had selected expression in the nucleus.
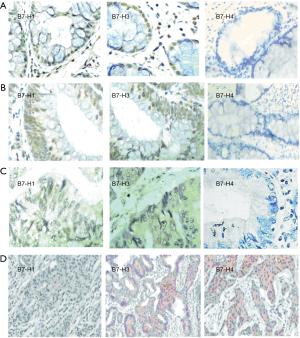
The expression of B7 family negative costimulatory molecules in the nucleus of CRC
B7 family molecules were also expressed in the cell membrane and the cytoplasm of colorectal carcinoma. The effect of their expression on tumor cells and microenvironment is not known yet. Therefore, the clinical significance of the expression of B7 family molecules in the nucleus of colorectal carcinoma and the regulation mechanism of the occurrence and development of colorectal carcinoma will be the focus of our further research direction.
Correlativity between B7-H1 molecule expression and clinic pathological factors in CRC stroma and tumor foci
According to the expression levels of B7 family molecules, CRC tissues were divided into low expression and high expression (Table 2). Chi-square test statistical analysis showed that the expression level of B7-H1 in interstitial lymphocytes of colorectal carcinoma was unrelated to sex, age, tumor stage, differentiation, tumor location, lymph node metastasis, distant metastasis, Dukes’ stage, and whether or not it is a mucinous adenocarcinoma. In cancer foci, the expression level indicated by B7-H1 was unrelated to patient sex, age, distant metastasis, tumor location, tumor stage, lymph node metastasis, Dukes’ stage, as well as mucinous adenocarcinoma. It was only related to the degree of differentiation of the patient, which means poor B7-H1 expression may be an independent prognostic factor. Chi-square test statistical analysis indicated that the expression level of B7-H3 molecules in colorectal carcinoma foci was unrelated to patient sex, age, tumor location, differentiation, tumor stage, lymph node metastasis, distant metastasis, Dukes’ stage, as well as mucinous adenocarcinomas. The expression level of B7-H3 in colorectal tissue stromal lymphocytes was unrelated to patient sex, age, tumor location, tumor stage, differentiation, distant metastasis, Dukes’ stage, and mucinous adenocarcinoma. Only related to lymph node metastasis; high expression of B7-H3 in colorectal lymphocytes was prone to cause lymph node metastasis. Chi-square test statistical analysis indicated that the expression level of B7-H4 in interstitial lymphocytes of colorectal carcinoma was independent of patient gender, tumor location, tumor stage, differentiation degree, distant metastasis, and Dukes’ stage. It was related to patient age, lymph node metastasis, mucinous adenocarcinoma. The expression level of B7-H4 in the foci was unrelated to patient sex, age, differentiation, tumor location, or mucinous adenocarcinoma. However, it was related to tumor stage, distant metastasis, lymph node metastasis, and Dukes’ staging. These results suggest that high expression of B7-H4 in lymphocytes and colorectal carcinoma cells is closely correlated with the progression of colorectal carcinoma. B7-H4 molecules might take a part in the most important immunosuppressive effect of the immune escape phase.
Full table
The relationship between the co-expression of B7 family molecules and prognosis of patients with CRC
We performed a seven-year survival analysis using the Kaplan-Meier method and the log-rank test. In 98 CRC patients, the survival rate of patients with low B7-H1 expression in the tumor stroma and the foci was higher than that of the patients with high B7-H1 expression. This confirms that B7-H1 is an independent prognostic factor for CRC (Figure 3A). There was no significant correlation between survival time and B7-H3 expression in tumor stroma and tumor foci (Figure 3B). Expression of B7-H4 in the tumor stroma had higher a survival rate in patients with low B7-H4 expression than those with high B7-H4 expression (P=0.045), but there was no significant correlation observed in cancer foci (Figure 3C). Furthermore, analysis of co-expression of B7 family molecules and prognosis in 98 CRC patients found that 92.9% of CRC patients expressed B7 negative costimulatory molecules in different degrees. The prognosis was negatively correlated with the number of co-expressing B7 negative costimulatory molecules (Figure 3D).
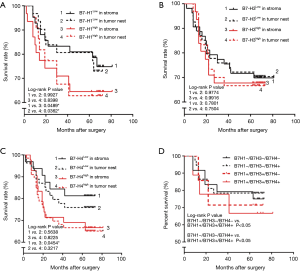
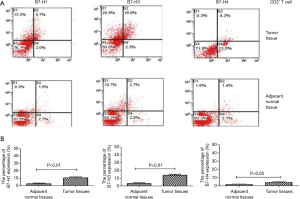
The relationship between CD3+ T cell infiltration and co-expression of B7-H1, B7-H3, and B7-H4 in CRC
We used flow cytometry analysis to measure the expression of B7 family molecules in CD3+ lymphocytes in colorectal carcinoma tissues and adjacent normal tissues (Figure 4A). The expression of its was significantly higher in CD3+ lymphocytes in colorectal carcinoma tissue than in adjacent normal tissues, and this difference was statistically significant (Figure 4B).
Discussion
The three molecules of B7 family, play a negative costimulatory effect during the carcinogenesis, and they negatively regulate the immune response by inhibiting the activation and proliferation of T cells (20). They were closely related to the immune escape mechanism of tumor cells, tumor proliferation, invasion, and metastasis. In the development of CRC, there were differences between the expression of these three negative co-stimulatory molecules in the benign and malignant lesions. The expression of B7-H1 and B7-H3 proteins were upregulated in colorectal polyps, whereas B7-H4 was only expressed during cancerous stages. The expression level of B7-H4 in colorectal tumorous interstitial lymphocytes was related to patient age, lymph node metastasis, and whether or not it was a mucinous adenocarcinoma (21). The expression level of B7-H4 in cancer foci was related to tumor stage, distant metastasis, lymph node metastasis, and Dukes’ staging. Survival analysis showed that the expression of B7-H1 and B7-H4 molecules in colorectal carcinoma was related to the survival rate of colorectal carcinoma patients, but there was no statistically significant correlation between the expression of B7-H3 and the survival rate of CRC patients. Our analysis of the co-expression of B7 family molecules in CRC and the prognosis of patients found that 92.9% of CRC patients expressed the B7 family of negative costimulatory molecules in different degrees. We also found that the prognosis is negatively correlated with the number of co-expressions of the negative costimulatory molecules in the B7 family. The correlation between these three negative co-stimulatory molecular expression levels with tumor pathological data and patient survival time has important clinical application value for clinical judgment of patient prognosis and guiding postoperative treatment.
B7 family molecules demonstrated a high degree of similarity in both mRNA and protein expression (22). In particular, mRNA was all expressed in various non-lymphoid tissues, including intestine, stomach, and lung. Investigation of the expression of B7 family molecules in various stages of CRC revealed that the B7 family negative molecules were barely expressed in normal colorectal tissue. The expression of B7 family negative molecules B7-H1 and B7-H3 began to increase in polyps and continued in the stages of adenoma, high-grade neoplasia, and CRC, but B7-H4 molecules were only expressed in the colorectal carcinoma stage. This suggests that B7-H1 and B7-H3 molecules could be potential warning molecules involved in initiating immune escape and causing CRC (8,21,23). These two molecules continue to be expressed as the tumor progresses, but the B7-H4 molecule only exerted negative regulation during the tumor immune escape phase (24). Therefore, B7-H1 and B7-H3 molecules might be potential targets for the early diagnosis and prevention of tumors, and the intervention of B7-H4 molecules is more likely to provide a new strategy for biological targeted therapy in CRC (22,25).
The expression of B7-H1, B7-H3, and B7-H4 molecules on the surface of infiltrating CD3+ T cells in colorectal carcinoma tissues was higher than that in adjacent normal tissues. This suggests that B7-H1, B7-H3, and B7-H4 molecules participate in immune escape. However, when the number of anti-tumor immune cells is sufficient, they can still control tumor progression, which also provides a favorable theoretical explanation for the treatment of adoptive immune cells (26). Further studies on the molecular mechanisms of the B7 family negative molecules could help us to understand the occurrence and development of colorectal carcinoma, and it may provide new intervention strategies for the early diagnosis and targeted treatment of colorectal carcinoma.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation Youth Project of China (No. 81502042), the grant from the Natural Science Foundation of Jiangsu Province (No. BK20171150).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.07.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical Review Committee of the Affiliated Hospital of Jiangnan University approved the experimental protocols. Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilyas M, Straub J, Tomlinson IP, et al. Genetic pathways in colorectal and other cancers. Eur J Cancer 1999;35:1986-2002. [Crossref] [PubMed]
- Stutman O. Immunodepression and malignancy. Adv Cancer Res 1975;22:261-422. [Crossref] [PubMed]
- Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol 2003;21:807-39. [Crossref] [PubMed]
- Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 1998;95:7556-61. [Crossref] [PubMed]
- Shi SJ, Ding ML, Wang LJ, et al. CD4+T cell specific B7-H1 selectively inhibits proliferation of negative T cells and Th17 differentiation in experimental autoimmune encephalomyelitis. Oncotarget 2017;8:90028-36. [Crossref] [PubMed]
- Zhi Y, Mou Z, Chen J, et al. B7H1 Expression and Epithelial-To-Mesenchymal Transition Phenotypes on Colorectal Cancer Stem-Like Cells. PLoS One 2015;10:e0135528 [Crossref] [PubMed]
- Deng R, Cassady K, Li X, et al. B7H1/CD80 interaction augments PD-1-dependent T cell apoptosis and ameliorates graft versus host disease. J. Immunol 2015;194:560-74. [Crossref] [PubMed]
- Ding Q, Lu L, Wang B, et al. B7H1-Ig fusion protein activates the CD4+ IFN-gamma receptor+ type 1 T regulatory subset through IFN-gamma-secreting Th1 cells. J Immunol 2006;177:3606-14. [Crossref] [PubMed]
- Kim JJ, Xu H, Haddad H, et al. B7H1 expression and association with clinical response to sunitinib therapy in patients with metastatic renal cell carcinoma (mRCC). BJU International 2013;103:7.
- Shanzhi W, Yiping H, Ling H, et al. The relationship between TTF-1 expression and EGFR mutations in lung adenocarcinomas. PLoS One 2014;9:e95479 [Crossref] [PubMed]
- Li Y, Huang J, Foley NM, et al. B7H3 ameliorates LPS-induced acute lung injury via attenuation of neutrophil migration and infiltration. Sci Rep 2016;6:31284. [Crossref] [PubMed]
- Xu F, Yi J, Wang F, et al. Involvement of soluble B7H3 in combination with the serum inflammatory cytokines interleukin17, 8 and 6 in the diagnosis of hepatocellular carcinoma. Oncol Lett 2017;14:8138-43. [PubMed]
- Zhao L, Xie C, Liu D, et al. Early Detection of Hepatocellular Carcinoma in Patients with Hepatocirrhosis by Soluble B7-H3. J Gastrointest Surg 2017;21:807-12. [Crossref] [PubMed]
- Inamura K, Yokouchi Y, Kobayashi M, et al. WITHDRAWN: Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer 2016;243:21-8. [PubMed]
- Li Y, Guo G, Jie S, et al. B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J Cancer 2017;8:816-24. [Crossref] [PubMed]
- Kasten BB, Arend R, Katre A, et al. B7-H3-targeted (212) Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl. Med. Biol 2017;47:23-30. [Crossref] [PubMed]
- Zhang P, Zhen C, Ning K, et al. Inhibition of B7-H3 reverses oxaliplatin resistance in human colorectal cancer cells. Biochem Biophys Res Commun 2017;490:1132-8. [Crossref] [PubMed]
- Mao Y, Chen L, Wang F, et al. Cancer cell-expressed B7-H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol Lett 2017;14:6177-83. [PubMed]
- Yang ZZ, Li L, Xu M, et al. Brain-derived neurotrophic factor involved epigenetic repression of UGT2B7 in colorectal carcinoma: A mechanism to alter morphine glucuronidation in tumor. Oncotarget 2017;8:29138-50. [PubMed]
- Liang M, Li J, Wang D, et al. T-cell infiltration and expressions of T lymphocyte co-inhibitory B7-H1 and B7-H4 molecules among colorectal cancer patients in northeast China's Heilongjiang province. Tumour Biol 2014;35:55-60. [Crossref] [PubMed]
- Zhou X, Mao Y, Zhu J, et al. TGF-β1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget 2016;7:67196-211. [PubMed]
- Peng HX, Wu W, Yang D, et al. Role of B7-H4 siRNA in Proliferation, Migration, and Invasion of LOVO Colorectal Carcinoma Cell Line. Biomed Res Int 2015;2015:326981 [Crossref] [PubMed]
- Yao Y, Ye H, Qi Z, et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin Cancer Res 2016;22:2778. [Crossref] [PubMed]
- Zhao H, Cheng Y, Dong S, et al. Down Regulation of miR-143 Promotes Radiation - Induced Thymic Lymphoma by Targeting B7H1. Toxicol Lett 2017;280:116-24. [Crossref] [PubMed]
- Lee YH, Martin-Orozco N, Zheng P, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res 2017;27:1034-45. [Crossref] [PubMed]
- Seaman S, Zhu Z, Saha S, et al. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017;31:501-15.e8. [Crossref] [PubMed]


