
Radiation therapy for superficial inguinal lymph node metastases from ovarian clear cell carcinoma and associated inguinal hernia
Introduction
Inguinal lymph node metastases (LNMs) from ovarian cancer (OC) are uncommon. Lymphangiogram and autopsy studies reportedly show that overall, approximately 3% of metastases from OC affect inguinal lymph nodes, and of these, 5% of inguinal LNMs are from the ovaries (1-3). The standard treatment for OC is cytoreductive surgery. Although superficial inguinal lymph node (SILN) metastases from OC are classified as distant, their treatment outcomes are better than those of distant metastases to other lymph nodes or organs; their control is therefore critical (4). To our knowledge, no reports regarding radiation therapy for SILN metastases from OC are available.
We herein describe a patient with SILN metastases from OC that were unresponsive to various chemotherapy regimens but responded to radiation therapy.
Case presentation
A 53-year-old Japanese woman presented to our hospital with a mass in her left groin that she had noticed gradually enlarging over the previous 5 months. A painless mass, adherent to surrounding tissues, was palpable in the left inguinal region. Computed tomography (CT) (Figure 1A) and magnetic resonance imaging (MRI) showed a 60-mm mass in the left SILN and giant pelvic masses indicative of bilateral ovarian tumors (Figure 1B).
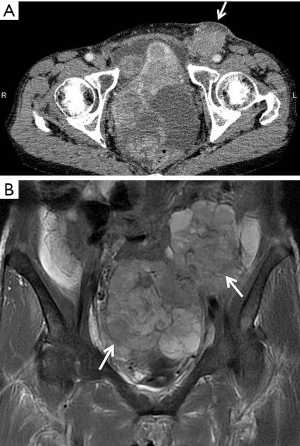
Serum cancer antigen (CA) 125 concentration was very high at 568 U/mL (normal range, <35 U/mL). The provisional diagnosis was bilateral OC and metastases to the left SILN. She underwent the standard primary surgical procedure of hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and pelvic lymphadenectomy. As the left SILN mass was large and unresectable because of its attachment to surrounding tissues, it was only biopsied.
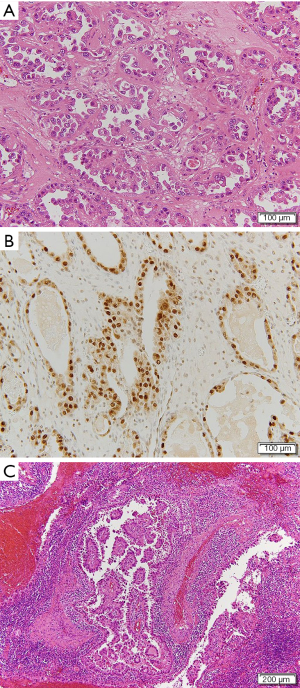
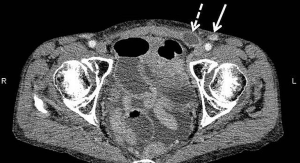
Histological examination of the resected specimen showed tubulocystic areas lined with cuboidal and hobnail cells in both ovaries (Figure 2A). Immunohistochemical analysis was positive for hepatocyte nuclear factor (HNF)-1β (Figure 2B). Based on these findings, the patient was diagnosed with clear cell carcinoma (CCC) of the ovaries. We also found tubulocystic areas lined with cuboidal and hobnail cells in the left SILN, which were consistent with the ovary tumor histological findings; accordingly, we diagnosed LNM from CCC of the ovaries (Figure 2C). Similar metastases were also found in the left supra-inguinal and left obturator lymph nodes. No evidence of malignancy was found in ascites or the greater omentum. The disease was classified as stage IV OC according to the 2014 International Federation of Gynecology and Obstetrics Ovarian Cancer Staging (5); thus, the patient received postoperative chemotherapy. Because the patient had recurrent paralytic ileus of the small intestine postoperatively, standard cycles of chemotherapy could not be administered. She therefore received the following chemotherapy regimens successively over approximately 12 months: 1 cycle of paclitaxel (180 mg/m2) plus carboplatin (AUC =6) (TC), 5 cycles of TC (paclitaxel 175 mg/m2) plus bevacizumab (Bev; 15 mg/kg), 4 cycles of Bev, and 4 cycles of irinotecan (100 mg/m2). At completion of TC, the left SILN metastases decreased somewhat in size, but regrew during maintenance Bev. We therefore changed the regimen to irinotecan, but the left SILN metastases did not decrease in size, as confirmed by positron emission tomography (PET)/CT. Serum CA-125 concentrations decreased postoperatively but increased thereafter to 121.6 U/mL, and further chemotherapy was thought to be ineffective. PET/CT showed no lesions other than the left SILN metastases, so we planned radiation therapy as local treatment. The left SILN metastases were defined as the gross tumor volume and the patient received three-dimensional conformal radiation therapy with 4-MV photon beams at a dose of 2 Gy/fraction (50 Gy in total). Intrapelvic irradiation was not administered because it was considered to have a high risk of intestinal adverse events in the presence of ileus. The sole adverse event attributable to radiation therapy was left grade 1 dermatitis (according to the Common Terminology Criteria for Adverse Events version 4.03). PET/CT performed 4 months after completing radiation therapy indicated a complete metabolic response according to the Response Evaluation Criteria in Solid Tumors version 1.1. At 104 days after completing radiation therapy, a new mass that was soft on palpation developed in the medial left inguinal region. A CT scan revealed ascites in a saccular inguinal hernia that involved the area from the left round ligament to the inguinal canal on the medial side of the shrinking SILN (Figure 3). The inguinal hernia had no symptoms and was observed without any treatment. At 77 days after its appearance, the inguinal hernia resolved, and no evidence of recurrent disease was observed over the 11 months after completing radiation therapy. Her subsequent serum CA-125 concentrations were within the normal limits and no further adverse events occurred.
Discussion
The standard therapy for advanced OC is cytoreductive surgery followed by systemic chemotherapy, the outcomes of which are better in patients with no residual lesions. However, the effectiveness of cytoreductive surgery for the rare phenomenon of inguinal LNM is unknown. Lymphadenectomy for inguinal LNMs may lead to postoperative complications; lower extremity lymphocele and lymphedema reportedly occur in 40–44% and 24–69% of all cases, respectively (6,7). In our patient, the SILN metastases were large and assessed as impossible to completely excise; therefore, they were only biopsied. Chemotherapy was subsequently administered in the hope that the SILN metastases would diminish in size, although ovarian CCC is more resistant to chemotherapy than other OC subtypes. In our patient, as the involved lymph nodes were resistant to various chemotherapy regimens administered over 12 months (as evidenced by their increasing size), radiation therapy was substituted. A search of published reports yielded 13 patients with inguinal LNMs from OC (8-17). All 13 of these patients underwent total excision of their lesions and adjuvant chemotherapy, and none of them received radiation therapy for their inguinal LNMs. Notably, most of those 13 patients had epithelial OC, whereas our patient had CCC. To our knowledge, this is the first case report of inguinal LNM from CCC.
OC can metastasize to lymph nodes via three routes: (I) the lymphatic vessels that accompany the ovaries to the paraaortic lymph nodes; (II) the subovarian plexus in the broad ligament to the obturator and pelvic lymph nodes; and (III) the round ligament to the external iliac and inguinal lymph nodes. In our patient, metastases were found in the left obturator and left supra-inguinal/inguinal lymph nodes; thus, metastasis had likely occurred via routes (II) and (III). We therefore considered including the intrapelvic region in the irradiation field, however, only the SILN mass was finally irradiated because the patient repeatedly experienced postoperative ileus and PET showed no accumulation outside of the SILN. No evidence of recurrent disease has been detected in the 11 months since completing radiation therapy. However, about 3 months after finishing radiation therapy, the patient unexpectedly developed an inguinal hernia between the SILN and inguinal canal, as shown by a CT scan. The causality of inguinal hernia after radiation therapy is unknown, but the mechanisms of connective tissue responses to radiation reportedly include vascular damage and fibrosis (18), and these mechanisms may promote inguinal hernia.
The role of radiation therapy for OC has not yet been established. However, recent studies have shown two satisfactory outcomes of radiation therapy. Prolonged survival was observed in patients who had received adjuvant whole-abdominopelvic irradiation in addition to adjuvant chemotherapy (19), and local control was achieved by irradiation at a median dose of 5,040 cGy in 82% of patients with recurrent lesions resistant to chemotherapy (20).
SILN metastases from OC are classified as distant, and have been recently reported to have approximately the same prognosis as that of stage III OC with paraaortic/pelvic LNMs. Local cancer control is therefore critical (4). A regimen of intrapelvic cytoreductive surgery, excision of inguinal LNMs (provided they are resectable), and radiation therapy may be appropriate for patients with OC with inguinal LNMs.
Acknowledgments
We thank Marla Brunker, from Edanz Group (
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.08). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Musumeci R, Banfi A, Bolis G, et al. Lymphangiography in patients with ovarian epithelial cancer: an evaluation of 289 consecutive cases. Cancer 1977;40:1444-9. [Crossref] [PubMed]
- Dvoretsky PM, Richards KA, Angel C, et al. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol 1988;19:57-63. [Crossref] [PubMed]
- Zaren HA, Copeland EM 3rd. Inguinal node metastases. Cancer 1978;41:919-23. [Crossref] [PubMed]
- Nasioudis D, Chapman-Davis E, Frey MK, et al. Should epithelial ovarian carcinoma metastatic to the inguinal lymph nodes be assigned stage IVB? Gynecol Oncol 2017;147:81-4. [Crossref] [PubMed]
- Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 2014;133:401-4. [Crossref] [PubMed]
- Gaarenstroom KN, Kenter GG, Trimbos JB, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer 2003;13:522-7. [Crossref] [PubMed]
- Buda A, Ghelardi A, Fruscio R, et al. The contribution of a collagen-fibrin patch (Tachosil) to prevent the postoperative lymphatic complications after groin lymphadenectomy: a double institution observational study. Eur J Obstet Gynecol Reprod Biol 2016;197:156-8. [Crossref] [PubMed]
- Shulman A, Ratzan WJ, Izenberg D. Primary ovarian cystadenocarcinoma: solitary metastasis to contralateral inguinal lymph node. Am J Obstet Gynecol 1953;66:197-8. [Crossref] [PubMed]
- McGonigle KF, Dudzinski MR. Endometrioid carcinoma of the ovary presenting with an enlarged inguinal lymph node without evidence of abdominal carcinomatosis. Gynecol Oncol 1992;45:225-8. [Crossref] [PubMed]
- Kehoe S, Luesley D, Rollason T. Ovarian carcinoma presenting with inguinal metastatic lymphadenopathy 33 months prior to intraabdominal disease. Gynecol Oncol 1993;50:128-30. [Crossref] [PubMed]
- Scholz HS, Lax S, Tamussino KF, et al. Inguinal lymph node metastasis as the only manifestation of lymphatic spread in ovarian cancer: A case report. Gynecol Oncol 1999;75:517-8. [Crossref] [PubMed]
- Manci N, Bellati F, Graziano M, et al. Ovarian cancer, diagnosed with PET, with bilateral inguinal lymphadenopathy as primary presenting sign. Gynecol Oncol 2006;100:621-2. [Crossref] [PubMed]
- Ang D, Ng KY, Tan HK, et al. Ovarian carcinoma presenting with isolated contralateral inguinal lymph node metastasis: a case report. Ann Acad Med Singapore 2007;36:427-30. [PubMed]
- Oei AL, de Hullu JA, Grefte JM, et al. An enlarged groin node as first manifestation of a malignancy: don't forget the ovaries. Eur J Obstet Gynecol Reprod Biol 2008;138:240-2. [Crossref] [PubMed]
- Mirrakhimov AE, Nwankwo N, Barbaryan A, et al. Left inguinal adenopathy two years after cytoreductive surgery: a rare sign of recurrence. Case Rep Oncol 2013;6:31-5. [Crossref] [PubMed]
- Yang XJ, Zheng FY, Xu YS, et al. Ovarian cancer initially presenting with isolated ipsilateral superficial inguinal lymph node metastasis: a case study and review of the literature. J Ovarian Res 2014;7:20. [Crossref] [PubMed]
- Metwally IH, Zuhdy M, Hassan A, et al. Ovarian cancer with metastatic inguinal lymphadenopathy: A case series and literature review. J Egypt Natl Canc Inst 2017;29:109-14. [Crossref] [PubMed]
- Wang J, Boerma M, Fu Q, Hauer-Jensen M. Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia 2006;10:502-6. [Crossref] [PubMed]
- Swenerton KD, Santos JL, Gilks CB, et al. Histotype predicts the curative potential of radiotherapy: the example of ovarian cancers. Ann Oncol 2011;22:341-7. [Crossref] [PubMed]
- Chundury A, Apicelli A, DeWees T, et al. Intensity modulated radiation therapy for recurrent ovarian cancer refractory to chemotherapy. Gynecol Oncol 2016;141:134-9. [Crossref] [PubMed]


