
Metastasis suppression and enhancement of anti-tumour immunity by targeting the FSTL1-DIP2A axis
Cancer immunotherapy, currently a subject of much interest in oncology, has in fact featured in medical literature for centuries. Physicians throughout history noted the coincidence between acute infection and tumour regression, and even used localized infection as rudimentary immunotherapy (1). Whilst today’s clinicians still seek to utilize the body’s natural defence mechanisms to treat cancer, we have become more aware of the challenges involved in immunotherapy. The immune system operates in a state of delicate balance—immune activation must be optimized to detect foreign pathogens whilst avoiding autoimmune attacks on the body’s own tissues. Cancer cells pose a problem for this self/non-self paradigm due to their origin from host tissue. Cancer recognition relies on both sufficient presentation of altered self-antigens by tumour cells, and the detection of such antigens by the immune system. At steady state, such antigens may go undetected as stringent checkpoint mechanisms exist to prevent indiscriminate immune activation. Major checkpoint proteins include cytotoxic T lymphocyte antigen (CTLA-4) and programmed cell death-1 (PD-1), both of which inhibit antigen specific T cell activation. During acute infection, these checkpoints are bypassed and immune cell activation proceeds. This, perhaps, represents the source of historical correlations between infection and regressing tumours—the heightened state of surveillance shifts the balance in favour of a particular antigen being recognized as non-self, thereby enhancing the ability of the immune system to detect cancer.
In the above interplay between the immune system and cancer, tumour cells themselves may play active roles in immune evasion. One strategy adopted for this purpose is the induction of immune checkpoint pathway molecules, such as CTLA-4 and PD-1, by cancer cells in order to block immune activation (2). The discovery of this mechanism, and the realization that targeting inhibitory checkpoint (IC) pathways could significantly inhibit tumour metastasis, led to the award of the 2018 Nobel Prize in Physiology and Medicine to James P. Allison and Tasuku Honjo. Successful clinical trials have also been carried out using monoclonal antibodies against CTLA-4 and PD-1 (3). However, despite the successes of IC inhibitors in immunotherapy, the technology is not universally safe and efficacious. Only a segment of patients respond to IC inhibitor therapy, and adverse consequences have been documented; haematological toxicity resulting from anti-CTLA-1/PD-1 is frequently reported, and immunotherapy-related death occurs in up to 2% of patients (4,5).
In this report, Kudo-Saito et al. (6) in 2018 discuss IC inhibitor therapy failure and present an alternative therapeutic strategy distinct from immune checkpoint pathways (Figure 1). IC inhibitors fail in the clinic for a number of reasons, including tumour heterogeneity, clonal selection for resistance and, most significantly, immune exhaustion. The latter frequently results from age-related immune deterioration; accumulated exposure to inflammatory stimuli with age promotes bone marrow dysfunction and immunosenescence, which may occur even in the absence of cancer. Previous work by this group has identified a key player in immune dysfunction—the secreted glycoprotein follistatin-like 1 (FSTL1). FSTL1, acting through its receptor DIP2A, directly increases tumour cell invasion potential, and also indirectly supports oncogenesis by stimulating the selective expansion of immunosuppressive mesenchymal stem cells (MSCs) that induce immune tolerance (7). This new study investigates the effects of targeting FSTL1 using monoclonal antibodies (mAbs) on anti-tumour immunity. A panel of anti-FSTL1 mAbs developed in-house was evaluated for anti-cancer properties. Treatment with these antibodies significantly reduced in vivo tumour growth and metastasis; intra-peritoneal injection of anti-FSTL1 increased the number of anti-tumor cytotoxic CD8+ T cells, decreased tumour mass, decreased tumour dissemination to bone marrow, and increased overall survival in mice. The anti-metastatic activity induced by anti-FSTL1 mAbs in lung metastasis models was superior to that produced by IC inhibitors when anti-FSTL1 therapy was administered on its own; when given in combination with IC inhibitors, anti-FSTL1 synergistically enhanced anti-tumour activity in multiple carcinoma models of tumor growth and metastasis. Notably, blocking FSTL1 effectively suppressed tumour growth even in aged mice. This finding is significant as it provide a means of mitigating the increased risk of cancer hyperprogression following IC inhibitor therapy in elderly patients (8).
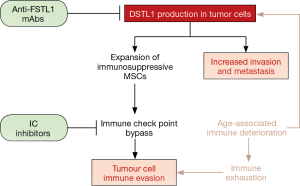
The effects of anti-FSTL1 therapy on reducing metastasis must be stressed. Metastatic dissemination of tumour cells to vital organs is the leading cause of cancer-related death (9). Solid tumours predominantly metastasize to the lungs, liver, and bones. Bone metastasis is a major contributor to mortality, and is often accompanied by symptoms such as severe pain, pathologic fractures, and hypercalcemia which drastically affect patients’ quality of life (10). In an earlier publication, the authors reported that tumour cells expressing the epithelial-to-mesenchymal marker SNAIL frequently metastasize to bone. Bone tropism in these cells is mediated by FSTL1, whose role in stimulating epithelial cell migration during wound repair (11) is hijacked to promote cancer cell invasion (12). As metastatic colonization is a multi-step process involving invasion of the bone microenvironment, immune evasion, and adaptation to the local niche, understanding the factors affecting bone tropism is essential to developing therapeutics against metastatic disease. Kudo-Saito et al. have performed just that, revealing the key role played by FSTL1 in bone tropism in their previous study, and highlighting here how FSTL1 may be therapeutically targeted to induce anti-cancer immunity where IC inhibitor treatment has failed. Whilst this technology is still unproven in clinical trials, it offers us another glimpse into the latent power of the immune system that we have yet to effectively deploy against cancer. The ancient art of immunotherapy is here to stay.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Jun Zhou, MD (Department of Nuclear Medicine, Zhongshan Hospital, Fudan University, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.25). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J 2003;79:672-80. [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017;390:1853-62. [Crossref] [PubMed]
- Petrelli F, Ardito R, Borgonovo K, et al. Haematological toxicities with immunotherapy in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 2018;103:7-16. [Crossref] [PubMed]
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
- Kudo-Saito C, Ishida A, Shouya Y, et al. Blocking the FSTL1-DIP2A Axis Improves Anti-tumor Immunity. Cell Rep 2018;24:1790-801. [Crossref] [PubMed]
- Kudo-Saito C, Fuwa T, Murakami K, et al. Targeting FSTL1 prevents tumor bone metastasis and consequent immune dysfunction. Cancer Res 2013;73:6185-93. [Crossref] [PubMed]
- Kim Y, Kim CH, Kim HS, et al. Hyperprogression after immunotherapy: Clinical implication and genomic alterations in advanced non-small cell lung cancer patients (NSCLC). J Clin Oncol 2018;36:9075. [Crossref]
- Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog 2013;18:43-73. [Crossref] [PubMed]
- Macedo F, Ladeira K, Pinho F, et al. Bone Metastases: An Overview. Oncol Rev 2017;11:321. [Crossref] [PubMed]
- Sundaram GM, Common JE, Gopal FE, et al. 'See-saw' expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature 2013;495:103-6. [Crossref] [PubMed]
- Sundaram GM, Ismail HM, Bashir M, et al. EGF hijacks miR-198/FSTL1 wound-healing switch and steers a two-pronged pathway toward metastasis. J Exp Med 2017;214:2889-900. [Crossref] [PubMed]


