
Risk factors and prediction of postoperative hypoparathyroidism among patients with papillary thyroid carcinoma
Introduction
Thyroid cancer is the most common endocrine malignancy, and surgery is the primary option for its treatment (1,2). Despite the wide application of meticulous capsular dissection (3), hypoparathyroidism is still a common complication of thyroid cancer surgery (4,5). Thyroid cancer has become the fastest growing type of carcinomas globally in recent years (6-8), and primary hospitals have come to take on the responsibility to perform more and more surgeries for thyroid cancer. This study included 93 patients with papillary thyroid carcinoma (PTC) who had undergone total or near-total thyroidectomy and central neck lymph node dissection (CND) from Ningbo No. 4 Hospital, China, and analyzed the incidence rate and risk factors of hypothyroidism with exploration on its predictive factors.
Methods
Inclusion and exclusion criteria
The criteria for including patients in the study are as follows: patients were admitted to Ningbo No. 4 Hospital from June, 2014 to January, 2016 and were newly diagnosed to have PTC with pathological confirmation; patients underwent total or near-total thyroidectomy as well as CND; patients were at 18 or above; patients had normal levels of parathyroid hormone (PTH) and serum calcium before surgery. Patients with history of neck surgery or parathyroid gland diseases were excluded. A total of 93 patients who met the criteria were included in the study.
Surgical procedures
All the patients had total or near-total thyroidectomy as well as ipsilateral or bilateral CND. For those who were confirmed by biopsy or highly suspected by imaging to have lymph node metastasis on either side of the neck, lateral lymph node dissection was performed. All surgeries were performed with meticulous capsular dissection in order to keep the parathyroid glands in situ as much as possible, while autotransplantation was performed when inadvertent parathyroidectomy or devascularization occurred.
Lab assays
All the patients were hospitalized for at least three days after surgery. They were monitored daily for their levels of PTH and serum calcium, magnesium, phosphorus, and albumin before and after surgery, and followed up at 1, 3, and 6 months after surgery for their levels of PTH and serum calcium. The reference ranges for all these biomedical indices in our lab are as follows: PTH, 15–65 ng/L; serum calcium, 2.00–2.60 mmol/L; serum magnesium, 0.70–1.10 mmol/L; serum phosphorus, 0.80–1.45 mmol/L, and albumin, 3.5–5.0 g/dL. Corrected calcium = serum calcium + 0.8×(4.0− serum albumin). Postoperative hypoparathyroidism was defined as a level of corrected calcium below 1.9 mmol/L regardless of presence of hypocalcemia or between 1.9 and 2.0 mmol/L with hypocalcemia. Permanent hypoparathyroidism was defined as the condition in the patients who still needed to take calcium and vitamin supplements after half a year post surgery. Patients were not given calcium supplements preventatively as a routine, while those with hypoparathyroidism were administered calcium supplements orally or through intravenous injection with vitamin D supplements and were immediately intervened for hypomagnesemia once found.
Statistical analysis
All statistical analysis was performed with SPSS 13.0 for Windows where a data set was created. Continuous variables were analyzed with χ2 or Fisher’s exact test, multivariate analysis was based on logistic regression, mean values were analyzed with independent t-test, ROC curve was used to evaluate the diagnostic value of postoperative PTH and serum calcium levels on hypoparathyroidism, and sensitivities, specificities, positive predictive values (PPV) and negative predictive values (NPV) were calculated with a 2×2 contingency table. Statistical significance was denoted by P<0.05.
Results
Postoperative hypoparathyroidism among the PTC patients
Among the 93 PTC patients, 18 were male (19.4%) and 75 were female (80.6%). The mean age of them was 45 years, ranging from 18 to 70. All the patients underwent total or near-total thyroidectomy; 43 (46.2%) had ipsilateral CND, and 50 (53.8%), bilateral; 19 patients (20.4%) had lateral lymph node dissection, and 15 had autotransplantation of parathyroid glands with transplantation of 1 gland in 13 patients and 2 in 2. According to the postoperative pathological reports, 40 patients had unilateral thyroid tumors (43%) and 53 (57%) bilateral; 47 (50.5%) had central lymph node metastasis with unilateral metastasis in 32 and bilateral 15; 24 (25.8%) had lateral lymph node metastasis, and 14 (15.1%) had extraglandular invasion by the primary tumor. In addition, 46 patients (49.5%) showed hypoparathyroidism after surgery, among whom 36 (38.7%) had various degrees of hypocalcemia and 2 (2.2%) had permanent hypoparathyroidism.
Clinicopathological risk factors of hypoparathyroidism
Univariate analysis showed that tumor size (P=0.034), extraglandular invasion (P=0.003), bilateral tumors (P=0.045), and bilateral CND (P=0.028) were significant risk factors of postoperative hypothyroidism among PTC patients, while in contrast, age, sex, lymph node metastasis, stage, and lateral lymph node dissection were not significant (P>0.05). Multivariate analysis showed that the independent significant risk factors of postoperative hypoparathyroidism were extraglandular invasion (OR, 19.30; 95% CI, 2.67–139.67; P=0.003) and bilateral CND (OR, 1.86; 95% CI, 1.38–9.06; P=0.044). These results of the uni- and multivariate analyses are shown in Table 1. In addition, the two patients with permanent hypoparathyroidism both had bilateral papillary carcinomas, and they both underwent total thyroidectomy and bilateral CND while one of them had obvious extraglandular invasion.
Table 1
| Variables | Number | Hypocalcemia, (%) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Gender | |||||||
| Male | 18 | 8 (44.4) | Ref. | 0.635 | Ref. | 0.776 | |
| Female | 75 | 38 (50.7) | 1.28 (0.46, 3.61) | 1.20 (0.34,4.24) | |||
| Age | |||||||
| ≥45 years | 44 | 18 (40.9) | Ref. | 0.118 | Ref. | 0.156 | |
| <45 years | 49 | 28 (57.1) | 1.93 (0.84, 4.40) | 1.035 (0.987, 1.084) | |||
| Tumor size | |||||||
| ≤1 cm | 66 | 28 (42.4) | Ref. | 0.034 | Ref. | 0.101 | |
| >1 cm | 27 | 18 (66.7) | 2.71 (1.06, 6.90) | 3.17 (0.80, 12.56) | |||
| Perithyroidal extension | |||||||
| Absent | 79 | 34 (43.0) | Ref. | 0.003 | Ref. | 0.003 | |
| Present | 14 | 12 (85.7) | 7.94 (1.67, 37.9) | 19.30 (2.67, 139.67) | |||
| Lymph node metastasis | |||||||
| No | 40 | 19 (47.5) | Ref. | 0.742 | Ref. | 0.190] | |
| Yes | 53 | 27 (50.9) | 1.15 (0.51, 2.61) | 0.45 (0.14, 1.48) | |||
| Bilaterality | |||||||
| Unilateral | 40 | 15 (37.5) | Ref. | 0.045 | Ref. | 0.325 | |
| Bilateral | 53 | 31 (58.5) | 2.35 (1.01, 5.44) | 2.01 (0.50, 8.06) | |||
| Clinical stage | |||||||
| I, II | 63 | 28 (44.4) | Ref. | 0.161 | Ref. | 0.444 | |
| III, IV | 30 | 18 (60.0) | 2.33 (0.73, 7.50) | 2.33 (0.73, 7.50) | |||
| CND | |||||||
| Ipsilateral | 43 | 16 (37.2) | Ref. | 0.028 | Ref. | 0.044 | |
| Bilateral | 50 | 30 (60.0) | 2.53 (1.10, 5.85) | 1.86 (1.38, 9.06) | |||
| Lateral neck dissection | |||||||
| No | 74 | 38 (51.4) | Ref. | 0.472 | Ref. | 0.621 | |
| Yes | 19 | 8 (42.1) | 0.69 (0.25, 1.91) | 0.79 (0.31, 2.01) | |||
PTC, papillary thyroid carcinoma; OR, odds ratio; CI, confidence interval; Ref., reference; CND, central neck dissection.
Analysis of the biochemical indices of hypoparathyroidism
There was no significant difference in the corrected level of preoperative serum calcium between the patients with hypoparathyroidism and those without, but the corrected level of postoperative serum calcium when they were hospitalized had an average nadir of 1.75 mmol/L for the former and 2.12 mmol/L for the latter, the difference of which showed statistical significance (P<0.001). The serum calcium levels of those with hypoparathyroidism reached the normal range 1 month after surgery and showed no difference from the levels of those without hypoparathyroidism except for two patients who had permanent hypoparathyroidism as their levels of serum calcium, 1.82 and 1.74 mmol/L, failed to reach the normal range 6 months after surgery. In addition, the preoperative PTH levels for the patients with hypoparathyroidism and those without were 39.71 and 43.77 ng/L, respectively, and they were not significantly different (P=0.125). However, the two groups showed significant difference in PTH levels at day 1, day 3, and 1 month after surgery (P<0.05) with the hypoparathyroidism patients having lower levels of PTH. Six months after surgery, the two groups no longer had significant difference in the PTH levels (P=0.613). These results are shown in Table 2.
Table 2
| Variables | Hypothyroidism (n=46) | Non-hypothyroidism (n=47) | P value |
|---|---|---|---|
| Corrected serum calcium (mmol/L) | |||
| Preoperative | 2.32±0.07 | 2.31±0.08 | 0.487 |
| Day 1 after operation | 2.08±0.18 | 2.17±0.18 | 0.012 |
| Postoperative nadir | 1.73±0.14 | 2.12±0.16 | <0.001 |
| 1 month after operation | 2.31±0.17 | 2.30±0.12 | 0.727 |
| 6 months after operation | 2.29±0.18 | 2.28±0.09 | 0.723 |
| PTH (ng/L) | |||
| Preoperative | 39.71±11.27 | 43.77±13.82 | 0.125 |
| Day 1 after operation | 8.51±5.16 | 21.39±10.69 | <0.001 |
| Day 3 after operation | 10.22±7.59 | 19.78±8.19 | <0.001 |
| 1 month after operation | 20.25±9.54 | 26.63±5.79 | 0.027 |
| 6 months after operation | 22.52±7.74 | 27.30±11.03 | 0.613 |
PTH, parathyroid hormone.
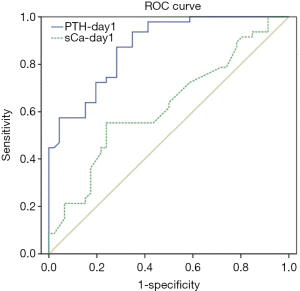
As shown in Table 2, the average corrected levels of serum calcium of those with hypoparathyroidism and those without were 2.08 and 2.17 mmol/L, respectively (P=0.012) on the first day after surgery (day 1). In fact, among the 46 patients with hypoparathyroidism, 35 (76.1%) had serum calcium levels within the normal range on that day, and 11 (23.9%) had levels lower than 2.11 mmol/L. The two patients with permanent hypoparathyroidism had 2.11 and 1.80 mmol/L of serum calcium, respectively, on day 1 after surgery. In addition, the average PTH levels of those with hypoparathyroidism and those without were 8.51 and 21.39 ng/L, respectively, on day 1, which had significant difference. The two patients with permanent hypoparathyroidism had PTH levels at 1.20 and 5.92 ng/L, respectively, on day 1, which showed a large-scale decrease from their preoperative levels. Then the ROC curve was drawn to test how well the PTH and the corrected serum calcium levels on day 1 could predict hypoparathyroidism, and the area under curve (AUC) for PTH was 0.875 and 0.622 for calcium, suggesting that PTH had a better predictive performance than corrected serum calcium (Figure S1). We then tested the predictive performance of the PTH levels on day 1 with several cutoff values. As shown in Table 3, when 25 ng/L was used as cutoff, the NPV was as high as 100%; when 5 ng/L was used as cutoff, the PPV was as high as 100%.
Table 3
| Variables | Cutoff values of PTH-day 1 (%) | |||
|---|---|---|---|---|
| 5 ng/L | 10 ng/L | 15 ng/L | 25 ng/L | |
| Sensitivity | 32.6 | 63.0 | 93.5 | 100 |
| Specificity | 100 | 93.6 | 57.4 | 27.7 |
| PPV | 100 | 90.6 | 68.3 | 57.5 |
| NPV | 60.3 | 72.1 | 90.0 | 100 |
PTH, parathyroid hormone; PPV, positive predictive value; NPV, negative predictive value.
Discussion
In the recent decade, thyroid cancer was the fastest growing malignancy, which could be mostly attributed to the increase in PTC (9). To deal with the growth, primary hospitals have to take on the responsibility to perform more and more surgeries for PTC treatment, and it has brought new challenges (10). The parathyroid glands are small in volume, vary in number and location, and have vulnerable vascular supply; therefore, hypoparathyroidism is the most common postoperative complication for PTC patients, which, if permanent, may severely compromise the life quality of the patients (11). Unfortunately, malignant thyroid tumors usually have to be treated with total thyroidectomy and CND, and the procedure adds to the incidence rate of hypoparathyroidism. Studies have shown that the incidence rate of thyroid cancer postoperative complications is associated with the number of surgeries performed by the surgeon and unexperienced surgeons have higher rates of postoperative complications (12). This study explored the risk of hypoparathyroidism and its predictive factors by analyzing 93 PTC patients from Ningbo No. 4 Hospital who had undergone total or near-total thyroidectomy and CND.
The incidence rate of hypoparathyroidism after thyroidectomy varied in various studies, which ranged from 1.7% to 68%, while most studies reported it to be between 20% and 30%, and it was associated with disease type, surgery procedure, and diagnostic standards (13,14). Higgins et al. studied 104 patients with total thyroidectomy, and found 23 (22.1%) developed hypoparathyroidism with 2 permanent cases. However, only 52 patients in the study had malignant thyroid tumors and 28 underwent paratracheal lymph node dissection (15). In our study, the incidence rate of postoperative hypoparathyroidism was 49.5%, higher than what was reported in most studies, which were all around 30%. One reason for the high rate was that all the patients in this study underwent total or near-total thyroidectomy and CND, and 53.8% had bilateral CND. More anatomical procedures, especially CND, undoubtedly led to higher incidence of inadvertent parathyroidectomy and devascularization. According to Roh et al., the incidence rate of hypoparathyroidism was 30.1% for the patients undergoing total thyroidectomy, and if CND was added to the surgery, the incidence rate would rise to 63.2% with 3.3% being permanent (16). Some scholars from South Korea reported 817 patients with thyroid cancer who had all undergone total thyroidectomy and CND; 42.2% of the patients showed postoperative hypocalcemia (17). In our study, both univariate and multivariate analyses showed that bilateral CND was a significant risk factor for hypoparathyroidism, which occurred in 60% of the patients who had undergone the procedure, considerably higher than the rate, 37.2%, among those with ipsilateral CND. As a matter of fact, the two patients with permanent hypoparathyroidism both had bilateral CND. These results showed that particular care must be taken when bilateral CND were to be performed and it had better be performed by experienced surgeons. In addition, multivariate analysis showed that aside from bilateral CND, extraglandular invasion was also an independent risk factor for hypoparathyroidism. This was probably because extraglandular invasion makes it difficult for surgeons to preserve the parathyroid glands in situ and for the glands to have blood supply; furthermore, extraglandular invasion may lead to lymph node metastasis, which subjects patients to bilateral CND; and these results echoed the study by Wang et al. (18). However, we did not find that there was no correlation between the number of residual parathyroid and extraglandular invasion in our study (data not shown). In addition, tumor size and bilateral tumors were both significant factors in the univariate analysis, but not in the multivariate analysis. Moreover, present study also showed the potential correlation between treatment of total thyroidectomy and CND with less neck surgery, which provides information to help surgeons define the risks of aggressive surgery and balance against the potential oncological benefit.
Once post-operative haemorrhage is no longer a risk, hypocalcemia, caused by hypoparathyroidism, becomes the major factor that prevents patients to be discharged from hospital (19). However, the calcium level decreases fairly slowly, often reaching the nadir at 48 hours after surgery; in fact, only 23.9% of the hypoparathyroidism patients in our study showed a below-normal calcium level. Therefore, the serum calcium level cannot be used as an early prognostic factor. When it comes to PTH, its half-life is only 2–5 minutes and it can be measured quickly and accurately; in addition, many studies have shown that early postoperative PTH levels are strongly associated with postoperative hypocalcemia (20). Therefore, the perioperative PTH level has been taken as the most effective marker to predict hypoparathyroidism although the prediction is not 100% accurate. After all, if the postoperative PTH level is normal, hypocalcemia, if any, is slight and self-restrictive (21). Our study showed that the PTH level on day 1 had an apparently better predictive performance for hypoparathyroidism, with the AUC as large as 0.875, compared to the serum calcium level on the same day. Therefore, PTH, as an early indicator of hypoparathyroidism, may signal early supplement of calcium for patients and help them be discharged from hospital early.
Conclusions
Overall, hypoparathyroidism is a common complication for PTC patients undergoing total or near-total thyroidectomy and CND. Extraglandular invasion and bilateral CND are two independent risk factors for it. The PTH level is an early, reliable indicator to predict hypoparathyroidism.
Acknowledgments
Funding: The work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.02.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ethics Committees of Zhejiang University School of Medicine). The number of ethics approval was 2018-450. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doubleday A, Sippel RS. Surgical options for thyroid cancer and post-surgical management. Expert Rev Endocrinol Metab 2018;13:137-48. [Crossref] [PubMed]
- Zhang K, Li C, Liu J, et al. DNA methylation alterations as therapeutic prospects in thyroid cancer. J Endocrinol Invest 2019;42:363-70. [Crossref] [PubMed]
- Su A, Gong Y, Wu W, et al. Effect of autotransplantation of a parathyroid gland on hypoparathyroidism after total thyroidectomy. Endocr Connect 2018;7:286-94. [Crossref] [PubMed]
- Iglesias P, Diez JJ. Endocrine Complications of Surgical Treatment of Thyroid Cancer: An Update. Exp Clin Endocrinol Diabetes 2017;125:497-505. [Crossref] [PubMed]
- Carling T, Udelsman R. Thyroid cancer. Annu Rev Med 2014;65:125-37. [Crossref] [PubMed]
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Parker WA, Edafe O, Balasubramanian SP. Long-term treatment-related morbidity in differentiated thyroid cancer: a systematic review of the literature. Pragmat Obs Res 2017;8:57-67. [Crossref] [PubMed]
- Schmid D, Ricci C, Behrens G, et al. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 2015;16:1042-54. [Crossref] [PubMed]
- Sun G, Qiu L, Cheng Z, et al. Association of the characteristics of B- and T-cell repertoires with papillary thyroid carcinoma. Oncol Lett 2018;16:1584-92. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Teshima M, Otsuki N, Morita N, et al. Postoperative hypoparathyroidism after total thyroidectomy for thyroid cancer. Auris Nasus Larynx 2018;45:1233-8. [Crossref] [PubMed]
- Hauch A, Al-Qurayshi Z, Randolph G, et al. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol 2014;21:3844-52. [Crossref] [PubMed]
- Quiros RM, Pesce CE, Wilhelm SM, et al. Intraoperative parathyroid hormone levels in thyroid surgery are predictive of postoperative hypoparathyroidism and need for vitamin D supplementation. Am J Surg 2005;189:306-9. [Crossref] [PubMed]
- Cavicchi O, Piccin O, Caliceti U, et al. Accuracy of PTH assay and corrected calcium in early prediction of hypoparathyroidism after thyroid surgery. Otolaryngol Head Neck Surg 2008;138:594-600. [Crossref] [PubMed]
- Higgins KM, Mandell DL, Govindaraj S, et al. The role of intraoperative rapid parathyroid hormone monitoring for predicting thyroidectomy-related hypocalcemia. Arch Otolaryngol Head Neck Surg 2004;130:63-7. [Crossref] [PubMed]
- Roh JL, Park CI. Intraoperative parathyroid hormone assay for management of patients undergoing total thyroidectomy. Head Neck 2006;28:990-7. [Crossref] [PubMed]
- Lee YM, Cho JY, Sung TY, et al. Clinicopathological risk factors and biochemical predictors of safe discharge after total thyroidectomy and central compartment node dissection for thyroid cancer: a prospective study. Int J Endocrinol 2015;2015:214525. [Crossref] [PubMed]
- Wang J, Gu J, Han Q, et al. Value of intraoperative parathyroid hormone monitoring in papillary thyroid cancer surgery: can it be used to guide the choice of operation methods? Int J Clin Exp Med 2015;8:7778-85. [PubMed]
- Aqtashi B, Ahmad N, Frotzler A, et al. Risk factors for hypocalcaemia after completion hemithyroidectomy in thyroid cancer. Swiss Med Wkly 2017;147:w14513. [PubMed]
- Mathur A, Nagarajan N, Kahan S, et al. Association of Parathyroid Hormone Level With Postthyroidectomy Hypocalcemia: A Systematic Review. JAMA Surg 2018;153:69-76. [Crossref] [PubMed]
- Guidelines AES. 06/01 Group. Australian Endocrine Surgeons Guidelines AES06/01. Postoperative parathyroid hormone measurement and early discharge after total thyroidectomy: analysis of Australian data and management recommendations. ANZ J Surg 2007;77:199-202. [Crossref] [PubMed]


