
Transcatheter embolization of hepatocellular carcinoma with epirubicin-loaded DC beads in Chinese patients
Introduction
Hepatocellular carcinoma (HCC) is the most common cancer worldwide, and is ranked as the third leading cause of cancer-related death (1). Current therapeutic options are based on the Barcelona clinic liver cancer (BCLC) staging system, integrating tumor characteristics and performance status with liver function. Surgical resection and liver transplantation are currently the accepted treatment choice in patients who have early-stage HCC with decompensated cirrhosis.
Transcatheter chemoembolization (TACE) is the most commonly used palliative treatment for patients with unresectable HCC (2,3). The principle of conventional TACE (cTACE) is the synergistic effect of cytotoxic chemotherapy and ischemia. Intra-arterial chemotherapeutic agents are mixed with lipiodol, which causes cytotoxic damage to the tumor cells, as well as embolization of the supplying blood vessels by gelatin or Gelfoam particles, resulting in ischemia (4). TACE is already recommended as the standard therapy for intermediate HCC patients according to the current guidelines, allowing for cTACE to combine with embolizing particles for chemotherapy drug delivery. Although cTACE is generally applied in HCC treatment, the systemic toxicity of chemotherapy after cTACE is significant (5,6). For optimal therapeutic effect, higher doses of the intra-arterial chemotherapeutic agent need to be retained within the tumor.
Furthermore, a chemotherapeutic drug that is released can reduce systemic side effects, and drug-eluting beads (DEBs) have been developed with these objectives in mind (5). DC beads (BTG International Ltd., UK) can load and release doxorubicin hydrochloride in a controlled manner (5). A previous study reported that TACE using beads loaded with doxorubicin (DEBDOX) induced significantly fewer drug-related side effects than cTACE (7). Moreover, DEB-TACE is reported to be safer and more effective than cTACE in HCC treatment (8-10). Few studies have been performed to evaluate the safety and efficacy of DEB-TACE when compared to cTACE for HCC.
The purpose of this study was to investigate the safety and efficacy of DEB-TACE treatment by DC bead when compared to cTACE for HCC in Chinese patients, as well as to determine the predicting factors for treatment response.
Methods
Patients and specimen characteristics
Seventy-four HCC patients were treated with TACE with DEBs and 80 HCC patients were treated with cTACE at our institution over a 1 and a half year period. Patients were considered for transarterial therapy if they exhibited unresectable HCC (determined by transplant surgery) and met the following criteria: (I) diagnosed with HCC according to the AASLD criteria (American Association for the Study of the Liver Diseases) (11); (II) aged 20–75 years old; (III) Child-Pugh stage A or B (score of no more than 7); (IV) ECOG score (Eastern Cooperative Oncology Group) of 0–2; and (V) without intrahepatic arterial-portal fistula or intrahepatic arteriovenous fistula. The exclusion criteria for this study were as follows: (I) Child-Pugh stage C or renal failure; (II) known allergy or contraindicated for the chemoembolization reagent used in this study; (III) intrahepatic arterial-portal fistula or intrahepatic arteriovenous fistula; (IV) hepatic encephalopathy; (V) uncontrolled ascites; (VI) life expectancy of less than 3 months; and (VII) pregnant or lactating women.
Ethical approval
The study was performed according to the standards set by the 1975 Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2016-324). All participants provided signed informed consent.
Procedure
DC beads (300–500 µm, BTG International Ltd., UK) were loaded with 60 mg of epirubicin. The loading process used for the DEBs with epirubicin was as follows: using a tee joint, one vial of DC bead was used to mix the DEBs and epirubicin, and the mixed solution was shaken for 2 minutes and stored for 30 minutes at room temperature; next, the non-ionic contrast agent was added to the mixed solution.
Before the reagent mixing procedure, enhanced magnetic resonance imaging (MRI) of the liver was performed to detect any arteriovenous fistula, and to identify the arterial supply of the tumor. Additionally, all HCC embolization was conducted under topical anesthesia. Subsequently, the tumor arterial supply was catheterized by 2.8 French microcatheters (Boston Scientific, Watertown, MA, USA). After the microcatheters were inserted, the DC bead loaded with epirubicin was injected at the speed of 1 mL/min, with the injection procedure being discontinued when a stasis flow of contrast agent occurred. After 5 minutes, a second angiography was conducted, and embolization was continued if the tumor blood supply was still present. Once all tumors stains disappeared, the microcatheters were removed, and the embolization was completed. If one vial of DC beads was used and the embolization was not completed, another vial would be utilized to reach the embolization endpoint.
In the cTACE group, emulsions of lipiodol (5–20 mL) and DOX (20–30 mg) were thoroughly mixed by the pumping method, and were slowly injected into the tumor artery through a microcatheter under fluoroscopic monitoring to avoid reflux of lipiodol emulsion followed by the infusion of a gelatin sponge or embosphere. The TACE procedure was terminated when target blood flow interruption or tumor stain disappearance was observed.
Assessments
Clinical response post-treatment with TACE was assessed by MRI at 1–3 months post-TACE, according to the modified response evaluation criteria in solid tumors (mRECIST) criteria (12). The criteria contain the following 4 categories: complete response (CR), which was described as a disappearance of any intra-tumoral arterial enhancement in all target lesions; partial response (PR), defined as at least a 30% decrease in the sum of the diameters of viable target lesions compared with the baseline diameter of the target lesions; stable disease (SD), including any cases that did not meet the criteria for CR, PR or progressive disease (PD); PD, defined as an increase of at least 20% in the sum of the diameters of viable target lesions. The rate of overall response (OR) was calculated as the rate of CR plus PR.
Adverse events during and after treatment were recorded, in addition to all laboratory indices of the patients pre- and post-treatment. The severity of pain post-treatment with TACE was graded by the pain visual analog scale (VAS) (13), with the vomiting grade being determined by the frequency of vomiting episodes during the 24 h post-treatment. Also, liver injury was evaluated according to the grade of liver function, with the severity of liver toxicity being assessed by the Common Terminology Criteria for Adverse Events (CTCAE), developed by the National Cancer Institute (NCI) (14).
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). For the baseline characteristics analyses, the Chi-square and Fisher’s exact tests were used for the comparison of categorical variables, and Student’s t-test was used for the continuous variables. Data were presented as counts (%), mean ± standard deviation (SD), or median values (with 25th–75th percentiles). Univariate and multivariate logistic regression analyses were used for the assessment of predictors for tumor response. A P<0.05 was considered to be statistically significant.
Results
Baseline characteristics of HCC patients
Technical success was 100%. A total of 84 TACE procedures were performed in 74 patients (84 person-time patients) in the DEB-TACE group, and 102 TACE procedures were performed in 80 patients (102 person-time patients) in the cTACE group. Baseline characteristics of the patients are listed in Table 1. The mean patient age was 57.3±11.0 years in the DEB-TACE group and 55.6±11.9 years in the cTACE group, with this study consisting of 135 males and 19 females. Also, 62 patients (83.8%) in the DEB-TACE group and 69 (86.3%) in the cTACE group had cirrhosis. The median tumor distribution was 30.0% (10.0–40.0%) vs. 24.0% (8.0–37.0%), with the largest nodule being 6.2 (4.5–9.8) vs. 5.3 (2.5–8.8) cm. Additionally, the number of patients with ECOG PS of 0 and 1 was 65 (87.8%) and 9 (12.2%) in the DEB-TACE group, and 63 (78.8%), 17 (21.3%) in the cTACE group, respectively. The number of patients with Child-Pugh stage A and B was 62 (83.8%), and 12 (16.2%) in the DEB-TACE group, and 74 (92.5%), 6 (7.5%) in the cTACE group, respectively. In addition, 2 (2.7%), 21 (28.4%), and 51 (68.9%) patients were BCLC stage A, B, and C stage in the DEB-TACE group, respectively. Moreover, there was no significant differences observed in all baseline characteristics except the number of nodules >3 or ≤3 (Table 1).
Table 1
| Parameters | DEB-TACE | cTACE | P vaule |
|---|---|---|---|
| Patient (n) | 74 | 80 | – |
| Gender (male/female) | 67/7 | 68/12 | 0.296 |
| Age (mean ± SD, years) | 57.3±11.0 | 55.6±11.9 | 0.414 |
| Etiology | |||
| No hepatitis (n/%) | 2 (2.7) | 3 (3.75) | – |
| HBV (n/%) | 72 (97.3) | 75 (93.75) | – |
| HCV (n/%) | 0 (0.0) | 2 (2.5) | – |
| HIV (n/%) | 1 (1.4) | 0 (0.0) | – |
| Drink (n/%) | 35 (47.3) | 31 (38.8) | 0.284 |
| Cirrhosis (n/%) | 62 (83.8) | 69 (86.3) | 0.668 |
| Tumor distribution* (%) | 30.0 (10–40.0) | 24.0 (8.0–37.0) | 0.339 |
| Number of nodules | |||
| 1 (n/%) | 15 (20.3) | 9 (11.3) | 0.123 |
| >1 (n/%) | 59 (79.7) | 71 (88.7) | – |
| ≤3 (n/%) | 32 (43.3 | 22 (27.5) | 0.041 |
| >3 (n/%) | 42 (56.7) | 58 (72.5) | – |
| Largest nodule size (range, cm) | 6.2 (4.5–9.8) | 5.3 (2.5–8.8) | 0.312 |
| Portal vein invasion (n/%) | 43 (58.1) | 36 (45.0) | 0.104 |
| Hepatic vein invasion (n/%) | 9 (12.2) | 8 (10.0) | 0.669 |
| ECOG score | 0.133 | ||
| 0 (n/%) | 65 (87.8) | 63 (78.8) | |
| 1 (n/%) | 9 (12.2) | 17 (21.3) | |
| Child-Pugh stage | 0.093 | ||
| A (n/%) | 62 (83.4) | 74 (92.5) | |
| B (n/%) | 12 (16.2) | 6 (7.5) | |
| BCLC stage | 0.453 | ||
| A (n/%) | 2 (2.7) | 6 (7.5) | |
| B (n/%) | 21 (28.4) | 28 (35.0) | |
| C (n/%) | 51 (68.9) | 46 (57.5) | |
| AFP abnormal (n/%) | 52 (70.3) | 65 (81.3) | 0.111 |
| Previous treatment | |||
| cTACE (n/%) | 25 (33.8) | 39 (48.8) | 0.060 |
| Surgery (n/%) | 10 (13.5) | 16 (20.0) | 0.283 |
| Targeted therapy (n/%) | 8 (10.8) | 4 (5.0) | 0.179 |
*, Data are presented as median (25th–75th), mean ± SD or counts (%). DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE; SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona clinic liver cancer; AFP, alpha fetoprotein.
Treatment response after TACE treatment
The ORR was 71.6%, with 9 (12.2%) and 44 (59.5%) patients achieving CR and PR in the DEB-TACE group, respectively (Table 2). The ORR of the cTACE group was 62.5%, with 12 (15.0%) achieving CR and 38 (47.5%) achieving PR (Table 2). There was no significant difference in the ORR between the two groups (P=0.229) (Figure 1). In terms of clinical response, the ORR of treated nodules in the DEB-TACE group was 69.1% compared to 64.4% in the cTACE group, in which 18 (11.8%) and 87 (57.2%) patients had nodules that achieved CR and PR compared to 23 (10.2%) and 122 (54.2%) in the cTACE group (Table 3), respectively. There were also no significant differences between the two groups (P=0.969). Among the nodules achieving a PR in the DEB-TACE group, 40 (46.0%) had a necrosis rate higher than 80%, 39 (44.8%) had a necrosis rate ranging from 50–80%, and 8 (9.2%) had a necrosis rate less than 50% (Figure 2). Also, the mean necrosis rate was (61.28%±22.65%) in the DEB-TACE group and 52.35%±29.75% in the cTACE group (Table 4). The PR DEB-TACE group had a higher necrosis rate than the PR cTACE group (P=0.009) (Figure 2).
Table 2
| Parameters | DEB-TACE (n=74, %) | cTACE (n=80, %) | P value |
|---|---|---|---|
| CR | 9 (12.2) | 12 (15.0) | 0.608 |
| PR | 44 (59.5) | 38 (47.5) | 0.137 |
| ORR | 53 (71.6) | 50 (62.5) | 0.229 |
| SD | 11 (14.9) | 12 (15.0) | 0.981 |
| PD | 10 (13.5) | 18 (22.5) | 0.149 |
Data are presented as counts (%). HCC, hepatocellular carcinoma; DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE.
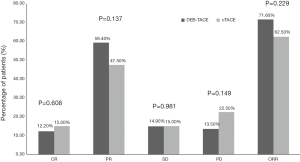
Table 3
| Parameters | DEB-TACE nodules (n=152, %) | cTACE nodules (n=225, %) | P value |
|---|---|---|---|
| CR | 18 (11.8) | 23 (10.2) | 0.620 |
| PR | 87 (57.2) | 122 (54.2) | 0.563 |
| ORR | 105 (69.1) | 155 (64.4) | 0.969 |
| SD | 25 (16.4) | 32 (12.5) | 0.554 |
| PD | 22 (14.5) | 38 (16.9) | 0.529 |
Data were presented as counts (%). DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE.
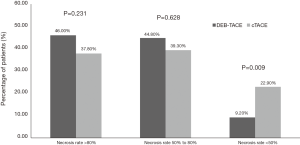
Table 4
| Parameters | DEB-TACE nodules (n=87, %) | cTACE nodules (n=122, %) | P value |
|---|---|---|---|
| Total necrosis rate (%) | 61.28±22.65 | 52.35±29.75 | 0.582 |
| Necrosis rate >80% | 40 (46.0) | 46 (37.8) | 0.231 |
| Necrosis rate 50% to 80% | 39 (44.8) | 48 (39.3) | 0.628 |
| Necrosis rate <50% | 8 (9.2) | 28 (22.9) | 0.009 |
Data are presented as mean ± standard deviation or counts (%). DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE.
Predictive factors analysis of ORR
Logistic regression analysis was performed to explore the predictive factors for tumor response in patients. As shown in Table 5, univariate logistic regression analysis determined that the number of nodules >3 (P=0.001), higher BCLC stage (P=0.047), portal vein invasion (P=0.031), previous cTACE (P=0.028), and previous surgery (P=0.009) were likely to be related with a worse ORR. The multivariate logistic regression analysis was performed with factors with a P<0.1. In the univariate logistic regression analysis, one factor, the number of nodules >3 (P=0.021), could independently predict a worse ORR.
Table 5
| Parameters | Univariate logistic regression | Multivariate logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | ||||
| Lower | Higher | Lower | Higher | ||||||
| Treatment (DEB-TACE vs. cTACE) | 0.131 | 1.683 | 0.857 | 3.304 | – | – | – | – | |
| Age ≥60 years | 0.350 | 1.380 | 0.703 | 2.710 | – | – | – | – | |
| Gender (male) | 0.430 | 0.647 | 0.220 | 1.907 | – | – | – | – | |
| Alcohol use | 0.742 | 1.124 | 0.559 | 2.259 | – | – | – | – | |
| Cirrhosis | 0.200 | 0.546 | 0.217 | 1.378 | – | – | – | – | |
| Tumor distribution ≥30% | 0.497 | 0.792 | 0.405 | 1.551 | – | – | – | – | |
| Number of nodules >1 | 0.135 | 0.450 | 0.158 | 1.282 | – | – | – | – | |
| Number of nodules >3 | 0.001 | 0.255 | 0.112 | 0.576 | 0.021 | 0.355 | 0.147 | 0.854 | |
| Largest nodule size >5 cm | 0.922 | 1.034 | 0.528 | 2.024 | – | – | – | – | |
| Portal vein invasion | 0.031 | 0.473 | 0.241 | 0.932 | 0.732 | 0.799 | 0.221 | 2.892 | |
| ECOG =0 (vs. 1) | 0.084 | 0.540 | 0.269 | 1.087 | – | – | – | – | |
| Child-Pugh stage A (vs. stage B) | 0.145 | 0.478 | 0.177 | 1.289 | – | – | – | – | |
| Higher BCLC stage (A + B vs. stage C) | 0.047 | 1.979 | 1.008 | 3.886 | 0.329 | 0.528 | 0.146 | 1.904 | |
| AFP abnormal | 0.280 | 0.637 | 0.282 | 1.443 | – | – | – | – | |
| Previous cTACE | 0.028 | 0.467 | 0.237 | 0.920 | 0.233 | 0.630 | 0.295 | 1.346 | |
| Previous surgery | 0.009 | 0.341 | 0.152 | 0.764 | 0.057 | 0.419 | 0.171 | 1.028 | |
| Previous targeted therapy | 0.935 | 1.054 | 0.302 | 3.675 | – | – | – | – | |
Data are presented as P value, OR and 95% CI. Factors affecting ORR achievement are determined by univariate logistic regression analysis. OR, odds ratio; CI, confidence interval; ORR, overall tumor response rate; DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona clinic liver cancer.
Liver function change before and after TACE
Liver function pre- and post-TACE were evaluated. The data showed that the CTCAE grades, based on baseline levels of ALB, TBIL, ALT, and AST, were only grade 0, 1, and 2, with grade 0 being the most prominent (Tables 6,7). Post-treatment, the liver toxicity grades increased compared to baseline (all P<0.001), with recovery time being within 1–3 months post-treatment (P=0.869, P=0.928, P=0.719, P=0.704 in the DEB-TACE group, and P=0.798, P=0.944, P=0.281, P=0.626 in the cTACE group, respectively).
Table 6
| Parameters | Baseline, TACEs (n=84) | 1-week post TACE, TACEs (n=84) | 1–3 months post TACE, TACEs (n=84) | P value* | P value# | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||||
| ALB (n) | 72 | 10 | 2 | 0 | 0 | 52 | 26 | 6 | 0 | 0 | 69 | 13 | 2 | 0 | 0 | <0.001 | 0.869 | ||
| TBIL (n) | 59 | 20 | 5 | 0 | 0 | 30 | 37 | 15 | 2 | 0 | 58 | 22 | 4 | 0 | 0 | <0.001 | 0.928 | ||
| ALT (n) | 70 | 11 | 3 | 0 | 0 | 28 | 30 | 19 | 6 | 1 | 68 | 13 | 2 | 0 | 0 | <0.001 | 0.719 | ||
| AST (n) | 57 | 25 | 2 | 0 | 0 | 22 | 42 | 12 | 7 | 1 | 61 | 22 | 1 | 0 | 0 | <0.001 | 0.704 | ||
*, baseline vs. 1-week post TACE; #, baseline vs. 1–3 months post TACE. DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE; ALB, albumin; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 7
| Parameters | Baseline, TACEs (n=102) | 1-week post TACE, TACEs (n=102) | 1–3 months post TACE, TACEs (n=102) | P value* | P value# | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||||
| ALB (n) | 87 | 12 | 3 | 0 | 0 | 65 | 31 | 6 | 0 | 0 | 85 | 15 | 2 | 0 | 0 | <0.001 | 0.798 | ||
| TBIL (n) | 71 | 24 | 7 | 0 | 0 | 37 | 45 | 17 | 3 | 0 | 70 | 26 | 6 | 0 | 0 | <0.001 | 0.944 | ||
| ALT (n) | 88 | 12 | 2 | 0 | 0 | 36 | 32 | 26 | 6 | 2 | 80 | 17 | 5 | 0 | 0 | <0.001 | 0.281 | ||
| AST (n) | 79 | 21 | 2 | 0 | 0 | 26 | 50 | 13 | 9 | 3 | 73 | 26 | 3 | 0 | 0 | <0.001 | 0.626 | ||
Data are presented as counts. Comparison among subgroups was analyzed by Wilcoxon signed-rank sum test, and P<0.05 was considered significant. *, baseline vs. 1-week post TACE; #, baseline vs. 1–3 months post TACE. cTACE, conventional transcatheter chemoembolization; cTACE, conventional TACE; ALB, albumin; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Common adverse events of the safety profile
As listed in Table 8, pain and vomiting were the most common adverse events post-TACE (≤24 h). The number of person-time patients presenting with light, moderate, and severe pain in the DEB-TACE group were 55 (65.5%), 26 (30.9%), and 1 (1.2%) in the cTACE group, respectively. For vomiting in the DEB-TACE group, 73 (86.9%) person-time patients did not vomit, with 11 (13.1%) presenting with grade 1 vomiting, compared to 25 (24.5%) and 77 (75.5%), respectively, in the cTACE group. Vomiting incidence in the cTACE group was higher than that of the DEB-TACE group (P=0.000). Only 7 person-time patients (8.3%) in the DEB-TACE group and 6 (5.88%) in the cTACE group presented with hypertension. In addition, fever was the most common adverse event post-treatment (24–72 h). In the DEB-TACE group, the majority of person-time patients had no fever (n=30, 35.7%), low-grade (n=23, 27.4%), and median-grade (n=23, 27.4%) fever, with only 8 person-time patients (9.5%) having high-grade fever. There were no significant differences in pain incidence, hypertension, and fever between the two groups.
Table 8
| Parameters | DEB-TACEs (n=84, %) | cTACEs (n=102, %) | P value |
|---|---|---|---|
| During and post operation (≤24 h) | |||
| Pain# | |||
| No pain# | 2 (2.38) | 2 (1.96) | 0.844 |
| Light pain# | 55 (65.5) | 65 (63.7) | 0.804 |
| Moderate pain# | 26 (30.9) | 32 (31.4) | 0.951 |
| Severe pain# | 1 (1.2) | 3 (2.94) | 0.628 |
| Vomiting* | |||
| No vomiting* | 73 (86.9) | 25 (24.5) | 0.000 |
| Grade 1* | 11 (13.1) | 77 (75.5) | 0.000 |
| Hypertension | 7 (8.3) | 6 (5.88) | 0.514 |
| Post operation (24–72 h) | |||
| Fever | |||
| No fever | 30 (35.7) | 33 (32.3) | 0.630 |
| Low-grade fever | 23 (27.4) | 28 (27.4) | 0.656 |
| Median-grade fever | 23 (27.4) | 29 (28.4) | 0.548 |
| High-grade fever | 8 (9.5) | 12 (11.8) | 0.623 |
Data are presented as counts (%). #, the severity of pain was calculated by visual analog scale (VAS) of pain: no pain =0; light pain =1–3; moderate pain =4–6; severe pain =7–10; *, Grade 1: times of vomiting =1–2; Grade 2: times of vomiting =3–5. DEB-TACE, transcatheter chemoembolization with drug eluting beads; cTACE, conventional TACE.
Discussion
HCC is the fifth most common form of cancer and the third leading cause of cancer-related death worldwide. Around 50% of worldwide HCC incidence originates in China, with HCC being the second leading cause of cancer-related death in China (1). While resection is the first-line curative treatment for liver cancer, the majority of patients are not candidates for resection, which makes TACE a standard treatment for unresectable, intermediate stage HCC patients. TACE has been clinically shown to prolong survival and can potentially benefit the patient quality of life.
In our study, DEB-TACE treatment for HCC patients showed good efficacy regarding the CR and PR rates, along with the ORR, compared to cTACE. The logistic regression analysis elucidated that BCLC stage, number of nodules, portal vein invasion, and cTACE and surgery history could be related to a worse ORR. Liver function of patients recovered within 3 months after a transient deterioration during the first-week post-TACE. DEB-TACE was well tolerable in patients with HCC, with only light to moderate toxicities observed in our study.
Based on previous research, DEB-TACE showed good efficacy in the treatment of HCC patients, with ORRs ranging from 35–84% (15-18). A previous study with DEB-TACE for HCC showed CR and PR rates of 58% and 31%, respectively, which was a higher CR rate than that found in our study (15). Most HCC patients were at BCLC A or B stage, but more than half of the patients in our study were at BCLC C stage, indicating that higher BCLC stage may affect TACE treatment response. While the study of Rahman et al. found that 17% of patients achieved a CR and 22% patients achieved a PR, the ORR was 39%, which was lower than what was found in our study (16). Moreover, a study performed in Korea found a PR rate of 28.3%, a CR rate of 32.1% and an ORR of 60.4% (18). The difference of tumor response between these studies might be a consequence of the different BCLC stages of hepatocarcinoma. DEB-TACE was used as the first-time treatment choice in some previous studies, which might have resulted in a higher tumor response rate after TACE, while the different sample sizes may also have caused different tumor response rates.
Although some criteria were developed specifically for the prognosis tumor response of HCC treatment, such as BCLC stage for risk classification and the Child-Pugh grade for evaluation of liver function with cirrhosis (19), predictive factors for tumor response of TACE are still not well established (20). Personalized treatment, including DEB-TACE, is becoming increasingly important due to the heterogeneity of liver cancer. Also, according to a prior study, C-arm computed tomography (CT) has been reported to predict the midterm tumor response (21). Another previous study illustrated that tumor enhancement of more than 50% and tumor heterogeneity are associated with CR after DEB-TACE (22). In our study, patients with higher BCLC stage and portal vein invasion had worse ORR, while the BCLC stage C patients had extremely poor tumor response rates. The BCLC stage of this study might be a consequence of the BCLC staging system assessment involving liver function, tumor distribution, and ECOG score, playing a prognostic role in HCC treatment (23). Kao et al. found that from 1,265 treatment-naive HCC patients, those with stages A2–A4 had markedly lower overall survival rates than those in stage 0 and A1. However, they did not compare the overall survival rates in different BCLC stages (24). Regarding predictive values of BCLC stage for tumor response after treatment, a previous study illustrated that the BCLC B and C stage of HCC patients had similar tumor response after chemoembolization (25).
In addition to the BCLC stage, our study found that multiple tumor nodules, along with previous cTACE and surgery, had worse ORR post-TACE. Multifocal tumors have been well accepted as an essential factor for predicting a worse prognosis in HCC patients (26-28). In addition to this, the number of nodules more than three has been reported as a predictive factor for poor survival after resection in patients of HCC (28). Results found from previous studies also indicate that the tumor nodules number may be of bad prognostic value, which is consistent with our findings. As for cTACE patients and surgery history in our study, those with a worse ORR might be this way because these patients, having previous cTACE treatment or HCC resection, were less sensitive to DEB-TACE treatment.
In our study, patient liver function was found to decline in the first week of post-treatment and recover rapidly within 1–3 months after TACE. Another study evaluating liver injury after DEB-TACE using imaging for 114 patients with HCC, found that the occurrence of severe liver injury, biliary injuries, intrahepatic biloma, and portal vein thrombosis to be 36.8%, 32.5%, 16.7%, and 4.4% respectively (29). Injury to the hepatic artery is another severe adverse event associated with DEB-TACE. In a previous study, among 54 HCC patients receiving DEB-TACE treatment, the incidence of grade 1, 2, and 3 hepatic artery injury was 13 (24.1%), 10 (18.5%) and 31 (57.4%) patients, respectively (30). Compared to these two previous studies, the hepatic injury in our study was relatively low, with the difference in this outcome possibly resulting from variation in the baseline global liver function in the previous reports. Moreover, the liver function of most patients recovered within 1–3 months in our study, and we found no significant differences between the DEB-TACE and cTACE group.
DEB-TACE has been found to be at least as tolerable as cTACE in previous related studies, with most toxicities presenting with low grades (16,31). To some extent, doxorubicin-related systemic toxicity has not been observed among patients with DEB-TACE, so DEB-TACE may be better tolerated than cTACE in HCC patients (32). A previous related study reported that for 7 out of 51 patients (13.7%) who presented with complications after DEB-TACE treatment for HCC, including liver abscess, gallbladder necrosis, severe pancreatitis, lung or cerebral embolism, the incidence of complication was relatively low (17). In this study, the most common complication intra and post-TACE were vomiting, fever and pain, among which most symptoms were light to moderate, which is also consistent with previous studies (16,17,31,32). Results from previous studies, as well as our own, demonstrate adequate safety with DEB-TACE treatment, and that DEB-TACE had a lower vomiting incidence than cTACE patients due to no doxorubicin-related systemic toxicity in our study.
Regarding the study’s limitations, this was a retrospective study that has a selection bias which could have influenced the results. The overall follow-up was a short, 1 to 3 months period, and thus the overall survival was not analyzed. Most patients had an HCC treatment history, which might have had an impact on the treatment outcome of DEB-TACE and cTACE.
Conclusions
In conclusion, DEB-TACE by DC bead was efficient and well-tolerated compared to cTACE in Chinese HCC patients. However, the present study showed no significant difference in the overall tumor response rate between DEB-TACE and cTACE. The BCLC stage, number of nodules, portal vein invasion, cTACE, and surgery history could possibly be predictive factors for a HCC treatment response.
Acknowledgments
Funding: The present work was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed according to the standards set by the 1975 Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2016-324). All participants provided signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-9. [Crossref] [PubMed]
- Imai N, Ishigami M, Ishizu Y, et al. Transarterial chemoembolization for hepatocellular carcinoma: A review of techniques. World J Hepatol 2014;6:844-50. [Crossref] [PubMed]
- Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81. [Crossref] [PubMed]
- Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007;5:1100-8. [Crossref] [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [Crossref] [PubMed]
- Baur J, Ritter CO, Germer CT, et al. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med 2016;8:69-74. [Crossref] [PubMed]
- Morimoto M, Kobayashi S, Moriya S, et al. Short-term efficacy of transarterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for hepatocellular carcinoma: comparison with conventional transarterial chemoembolization. Abdom Radiol (NY) 2017;42:612-9. [Crossref] [PubMed]
- Kucukay F, Badem S, Karan A, et al. A Single-Center Retrospective Comparison of Doxorubicin-Loaded HepaSphere Transarterial Chemoembolization with Conventional Transarterial Chemoembolization for Patients with Unresectable Hepatocellular Carcinoma. J Vasc Interv Radiol 2015;26:1622-9. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Swarm R, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw 2010;8:1046-86. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published May 28, 2009; Revised Version 4.03 June 14, 2010. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Manini MA, Sangiovanni A, Martinetti L, et al. Transarterial chemoembolization with drug-eluting beads is effective for the maintenance of the Milan-in status in patients with a small hepatocellular carcinoma. Liver Transpl 2015;21:1259-69. [Crossref] [PubMed]
- Rahman FA, Naidu J, Ngiu CS, et al. Conventional versus Doxorubicin-Eluting Beads Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma: a Tertiary Medical Centre Experience in Malaysia. Asian Pac J Cancer Prev 2016;17:4037-41. [PubMed]
- Arabi M, BenMousa A, Bzeizi K, et al. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol 2015;21:175-80. [Crossref] [PubMed]
- Liu YS, Ou MC, Tsai YS, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol 2015;16:125-32. [Crossref] [PubMed]
- Hucke F, Sieghart W, Pinter M, et al. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol 2014;60:118-26. [Crossref] [PubMed]
- Mähringer-Kunz A, Kloeckner R, Pitton M, et al. Validation of the Risk Prediction Models STATE-Score and START-Strategy to Guide TACE Treatment in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2017;40:1017-25. [Crossref] [PubMed]
- Syha R, Gatidis S, Grozinger G, et al. C-arm computed tomography and volume perfusion computed tomography (VPCT)-based assessment of blood volume changes in hepatocellular carcinoma in prediction of midterm tumor response to transarterial chemoembolization: a single center retrospective trial. Cancer Imaging 2016;16:30. [Crossref] [PubMed]
- Reis SP, Sutphin PD, Singal AG, et al. Tumor Enhancement and Heterogeneity Are Associated With Treatment Response to Drug-Eluting Bead Chemoembolization for Hepatocellular Carcinoma. J Comput Assist Tomogr 2017;41:289-93. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Kao WY, Chao Y, Chang CC, et al. Prognosis of Early-Stage Hepatocellular Carcinoma: The Clinical Implications of Substages of Barcelona Clinic Liver Cancer System Based on a Cohort of 1265 Patients. Medicine (Baltimore) 2015;94:e1929. [Crossref] [PubMed]
- Soydal C, Arslan MF, Kucuk ON, et al. Comparison of survival, safety, and efficacy after transarterial chemoembolization and radioembolization of Barcelona Clinic Liver Cancer stage B-C hepatocellular cancer patients. Nucl Med Commun 2016;37:646-9. [Crossref] [PubMed]
- Baffy G. Decoding multifocal hepatocellular carcinoma: an opportune pursuit. Hepatobiliary Surg Nutr 2015;4:206-10. [PubMed]
- Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med 2015;3:4. [PubMed]
- Goh BK, Chow PK, Teo JY, et al. Number of nodules, Child-Pugh status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J Gastrointest Surg 2014;18:1477-85. [Crossref] [PubMed]
- Monier A, Guiu B, Duran R, et al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol 2017;27:1431-9. [Crossref] [PubMed]
- Lee S, Kim KM, Lee SJ, et al. Hepatic arterial damage after transarterial chemoembolization for the treatment of hepatocellular carcinoma: comparison of drug-eluting bead and conventional chemoembolization in a retrospective controlled study. Acta Radiol 2017;58:131-9. [Crossref] [PubMed]
- Kalva SP, Pectasides M, Liu R, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol 2014;37:381-7. [Crossref] [PubMed]
- Nam HC, Jang B, Song MJ. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma. World J Gastroenterol 2016;22:8853-61. [Crossref] [PubMed]


