
Significant clinical response of advanced colorectal cancer to combination therapy involving capecitabine and adoptive cell transfer therapy: a case report
Introduction
Colorectal cancer (CRC) remains the fourth common cause of death. Because of the low examination rate for colonoscopy, many patients are diagnosed as advanced CRC. Presently, with systematic treatment including surgery, chemotherapy and radiation therapy, more and more patients are cured. While patients with advanced CRC, some with metastasis especially, still don’t have effective treatment (1). It is said that Asian and Pacific Islander has the highest overall 5-year survival rates. The 5-year survival rates for patients with a local stage CRC is 92.3%. Survival declines to 74.3% and 15.7% for patients diagnosed as regional and distant stage, respectively (2).
Fluoropyrimidine-based chemotherapy is accepted as the standard treatment for metastasis colorectal cancer (mCRC) (3). Capecitabine is an oral prodrug which is converted to its only active metabolite, 5-fluorouracil (5-FU), by thymidine phosphorylase (4). The fluoropyrimidine chemotherapy to the preoperative regimen reduces local recurrence (5), but does not improve survival (6), and has a negative effect on quality of life (7). Capecitabine combination with oxaliplatin is usually used for better therapeutic effect. While the addition of oxaliplatin doesn’t show positive effects in short-time result (8,9), but it has increased complications resulting in dose reductions and treatment interruptions (10,11). For the mCRC, the treatment is part of a palliative rather than curative treatment and the purpose of that is to prolong overall survival and to maintain quality of life (12). Other monoclonal anti-body (Mab) include cetuximab and panitumumab, both of which are epidermal growth factor receptor (EGFR) targeted Mab. They can regulate signal pathways related with cell survival, angiogenesis, cell migration and cellular invasion (13). Moreover, immunotherapy is a novel strategy for cancer therapy in recent years. Some clinical trials show the potential effectives for CRC patients (1).
Case presentation
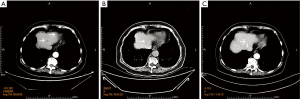
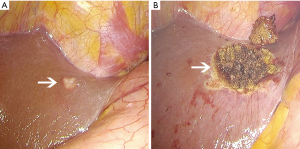
An 81-year-old woman was diagnosed as CRC in February 2017, and she complained the dark red colored bloody stool without any obviously causative factors. Her previous medical history included hypertension and osteoporosis, which had lasted 20 and 10 years respectively. Colonoscopy showed an irregular lump occupying 2/3 enteric cavity, which the colonoscopy couldn’t break through. The patient’s serum level of carcinoembryonic antigen (CEA) was 48.13 ng/mL, CA199 was 75.86 U/mL, and CA125 was 64.13 IU/mL. Computed tomography (CT) showed several hypodense lesions in liver, some of which were enhanced in enhancement CT (Figure 1). Evaluation of MR images revealed rectosigmoid colon was occupied. The case was suspected CRC (cT4aN1M1). Laparoscopic lower anterior resection and left liver metastatic carcinoma resecting were conducted in 20th, February, 2017 (Figure 2). The tumor was 5 cm-long and 4 cm-wide, cauliflower-like appearance, invading the serosa, and occupying 2/3 enteric cavity. A hepatic metastasis which located at the surface of left lobe was observed in the surgery and was resected at the same time, but some hypodense lesions in the CT images were unresectable (Figure 1A). The patient recovered well after the operation. The pathological diagnosis was differentiated adenocarcinoma invading to serosa with lymph node metastasis (4/13), and the resected lesion of left liver was confirmed as metastasis tumor. The surgical margin was free of tumor involvement. The test of immunohistochemistry showed EGFR(−), Ki67(high 80%, average 40%), MLH(+), PMS2(+), MSH2(+), MSH6(+).
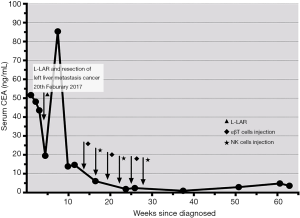
After released from the hospital, the patient takes capecitabine (1.5 g po bid, d1–14, 21 d/cycle) for further treatment. The patient’s serum level of CEA was dropped after the surgery, which was 19.52 ng/mL, but it increased dramatically 2 weeks later. Because of that, she enrolled immunotherapy six times from April to July 2017. αβT cells and NK cells were injected intravenously into the patient six courses. Peripheral blood mononuclear cells were harvested using centrifugation. Over 1×106 harvested cells were cultured with an immobilized antibody to CD3 and interleukin (IL)-2 for 14 days, and 7.54×109 (4.4×109–9.2×109 on average) lymphocytes were obtained. The cultured lymphocytes included 61%±15% of CD8+ T-cells, 30%±15% of CD4+ T-cells (CD4+: CD8+ ratio, 0.8 on average), and a small percentage of natural killer (NK) cells, indicating that the proliferation of CD8+ T-lymphocytes was much greater than that of CD4+ T-lymphocytes during the 2-week culture period. Over 7.54×109 αβ T-lymphocytes were injected intravenously into patients once every 3 weeks for 4.5 months (six cycles). After the first transfusion with αβT cells, the tumor biomarker, CEA, dropped obviously from 14.7 to 6.1 ng/mL. And it came to 1.9 ng/mL after four times treatment, which was back into normal range (<5 ng/mL) (Figure 3).
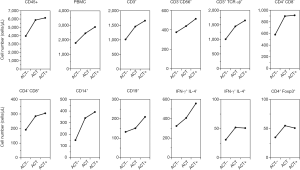
To reveal the detailed immunological status of this patient before and after adoptive cell transfer therapy, we used flow cytometry (FCM) to detect the intracellular or superficial markers of leukocytes. The detail method was described previously (14). We used Flow-Count™ fluorosphere internal standard beads to detect the absolute cell numbers. The OptiLyse C, Flow-Count beads, and monoclonal antibodies (mAbs) against CD3, CD4, CD8, CD14, CD19, CD45, CD56, TCR pan αβ, IFN-γ and IL-4 were purchased from Beckman Coulter (Brea, CA, USA). The result is shown diagrammatically in Figure 4. According to the figure, most kinds of lymphocytes were increased after infusion. The number of monocyte (CD14+), Killer T cell (CD3+ CD4− CD8+) and αβT cells (CD3+ TCR αβ+) rose by 259% (from 151 to 392 cells/µL), 159% (from 192 to 305 cells/µL) and 165% (from 1,007 to 1,657 cells/µL) respectively.
The CT images revealed the cancer didn’t progression or relapse after the immunotherapy. Compared with CT images before the surgery, the metastases are smaller and much harder to see in liver, and are now similar to the density of the normal liver (Figure 1B,C). With 19-month follow-up, no relapse was founded, and the serum level of CEA is within normal limits.
The severity of adverse effects was evaluated according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 4.0. The patient had slight anemia only, (Hgb 10.5 g/dL), which did not fulfill the definition of adverse effects according to NCI-CTC criteria. There were no other severe treatment-related adverse events and no treatment-related death were founded.
Discussion
Nowadays the main immunotherapy for CRC include tumor vaccines, cytokine treatment, mAbs, checkpoint therapies and adoptive cell transfer therapy (15). Previous study for metastasis CRC patients revealed chemoimmunotherapy can enhance proliferative response to colon carcinoma antigen and a significant reduction in suppressive regulatory T lymphocytes (Treg cells), resulting in enhanced T cells activities (16). It gives a prospect of combination of chemotherapy and immunotherapy. The conception of adoptive cell transfer therapy is that specific effector cells are directly infused within the cancer patient. Because most tumor cells express MHC class I-peptide, which can be recognized by antigen-specific CD8+ cytotoxic T lymphocytes (CTLs). Therefore, adoptive cell transfer of activated CTLs successfully used in patients with advanced cancer (17).
The treatment of this patient includes immunotherapy and chemotherapy. αβT cells and NK cells were injected intravenously into the patient. Capecitabine was taken as well. Tumors were measured after adoptive cell transfer therapy, and responses were evaluated according to the response evaluation criteria for solid tumors (RECIST), version 1.1 (18). The evaluation of responses was based on computed tomography measurements. Complete response (CR) was observed in this patient. The biggest lesion was 13.44 mm × 10.81 mm before the adoptive cell transfer therapy, while, it was unable to measure after the therapy. The CEA was back to the normal levels as well. It is confirmed combination therapy involving adoptive immunotherapy and chemotherapy for stage IV CRC is safe and feasible (19). In the research, therapy in each 21-day treatment cycle involved XELOX (130 mg/m2 of oxaliplatin on day 1 plus 1,000 mg/m2 of capecitabine twice daily on days 1–14), bevacizumab (7.5 mg/kg on day 1), and αβ T-lymphocytes (over 5×109 on day 18) cultured ex vivo with an immobilized antibody to CD3 and IL-2. The overall response rate was 83.3%. The median progression-free and overall survival durations were 567 and 966 days, respectively. The result provides a prospect for adoptive immunotherapy.
Immunotherapy has become a novel choice for solid cancer, though its effect still needs clinical trials to confirmed. PD-1, a check point inhibitor target, is one of the most popular strategies in immunotherapy. PD-1 inhibits T cells by down-regulating IL-2 expression through the PI3K/AKT pathway (20). While it is necessary to find reliable parameters for non-specific immunotherapy, such as adoptive cell transfer therapy. In previous works, we performed a detailed evaluation of the immunological status of 47 patients with advanced solid cancer, compared with 32 healthy subjects by FCM. And peripheral blood from 26 of 47 cancer patients was analyzed again after adoptive cell transfer therapy (14). Absolute numbers of T cells, several T cell subsets, B cells, and NK cells were significantly decreased in patients compared with healthy subjects. And adoptive cell transfer therapy increased the number of T cell subsets. This case provides an example for the conclusions. Before the adoptive cell transfer therapy, the absolute number of T cells is low, αβT cells especially, which are the majority of human T cells. After infusion cultured cells, the number of T cells increased, and it was much higher in 1 year assessment. This indicated adoptive cell transfer therapy can restore impaired T cells status. The patient was regarded as CR.
Adoptive immunotherapy still has some drawbacks (21), including a potential lack of immunologic memory, poor persistence of activated effector cells in patients and the time required to expand the cells. But, compared with chemotherapy, seldom serious complications of adoptive immunotherapy were recorded, except Parkhurst et al. reported three patients with mCRC were treated with autologous T lymphocytes genetically engineered to express a murine T cell receptor (TCR), which is against human CEA. All patients experienced profound decreases in serum CEA, however, a severe transient inflammatory colitis that represented a dose limiting toxicity was induced in all three patients (22).
The application of adoptive cell transfer therapy combined with capecitabine chemotherapy in patients with mCRC can ensure clinical efficacy with less complications. For some advanced CRC after primary site resection, the result of systematic treatment is still poor. Combination therapy involving adoptive immunotherapy and chemotherapy may be a feasible method to prolong the survival.
Acknowledgments
Funding: The present Case Report was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.02.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhen YH, Liu XH, Yang Y, et al. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother 2015;64:1083-93. [Crossref] [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Cassidy J. Adjuvant 5-fluorouracil plus levamisole in colon cancer: the plot thickens? Br J Cancer 1994;69:986-7. [Crossref] [PubMed]
- Walko CM, Lindley C. Capecitabine: a review. Clin Ther 2005;27:23-44. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Fiorica F, Cartei F, Licata A, et al. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta-analysis of literature data. Cancer Treat Rev 2010;36:539-49. [Crossref] [PubMed]
- Tiv M, Puyraveau M, Mineur L, et al. Long-term quality of life in patients with rectal cancer treated with preoperative (chemo)-radiotherapy within a randomized trial. Cancer Radiother 2010;14:530-4. [Crossref] [PubMed]
- Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638-44. [Crossref] [PubMed]
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [Crossref] [PubMed]
- Rodel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [Crossref] [PubMed]
- De Caluwe L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2013;CD006041. [PubMed]
- Sanchez-Gundin J, Fernandez-Carballido AM, Martinez-Valdivieso L, et al. New Trends in the Therapeutic Approach to Metastatic Colorectal Cancer. Int J Med Sci 2018;15:659-65. [Crossref] [PubMed]
- de Mello RA, Marques AM, Araujo A. Epidermal growth factor receptor and metastatic colorectal cancer: insights into target therapies. World J Gastroenterol 2013;19:6315-8. [Crossref] [PubMed]
- Noguchi A, Kaneko T, Naitoh K, et al. Impaired and imbalanced cellular immunological status assessed in advanced cancer patients and restoration of the T cell immune status by adoptive T-cell immunotherapy. Int Immunopharmacol 2014;18:90-7. [Crossref] [PubMed]
- Amin M, Lockhart AC. The potential role of immunotherapy to treat colorectal cancer. Expert Opin Investig Drugs 2015;24:329-44. [Crossref] [PubMed]
- Correale P, Cusi MG, Tsang KY, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol 2005;23:8950-8. [Crossref] [PubMed]
- Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today 1997;18:175-82. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Yoshida Y, Naito M, Yamada T, et al. Clinical Study on the Medical Value of Combination Therapy Involving Adoptive Immunotherapy and Chemotherapy for Stage IV Colorectal Cancer (COMVI Study). Anticancer Res 2017;37:3941-6. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Koido S, Ohkusa T, Homma S, et al. Immunotherapy for colorectal cancer. World J Gastroenterol 2013;19:8531-42. [Crossref] [PubMed]
- Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011;19:620-6. [Crossref] [PubMed]


