
Multiplex fluorescent immunohistochemistry quantitatively analyses microvascular density (MVD) and the roles of TGF-β signalling in orchestrating angiogenesis in colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumours (1). Angiogenesis provides oxygen and nutrients to and removes metabolic waste from tumour cells (2). Increased angiogenesis has been associated with neoplastic progression, metastasis and outcome in a number of malignancies (3). Angiogenesis is regulated by multiple growth factors, including VEGF, PDGF, and FGF (4,5). Microvascular density (MVD) is considered the ‘gold standard’ for measuring tumour angiogenesis (6). Many studies have shown that MVD could have potential value as a prognostic biomarker of tumours (7). CD31 is recognized as a biomarker of vascular endothelial cells (ECs) to represent MVD in tumour tissues (8).
The transforming growth factor (TGF)-β signalling pathway is an intriguing cytokine signalling pathway that exhibits dual activities in malignant tumour disease (9). TGF-β exhibits multifunction and acts as an important mediator of cancer angiogenesis (10,11). Research regarding the consequences of TGF-β signalling remains controversial. A large amount of data supports an early tumour-suppressive role for TGF-β. Additionally, TGF-β signalling within carcinoma cells can promote tumour progression and metastasis at late stages (12). Previously, we conducted a study to detect the multiple factors involved in TGF-β signalling in the tissues of patients with CRC by multiplex fluorescent immunohistochemistry (mfIHC) (9). Our results supported the idea that TGF-β exhibited tumour-suppressive effects at the early stage and promoted tumour progression at the late stage.
TGF-β consists of three isoforms (TGFB1, ~2, and ~3). TGFB1 is the most abundant and well-studied isoform (13). There are two typical TGF-β receptors, TGF-β type I and type II receptors (TGFBR1 and TGFBR2), with active serine/threonine kinase (14). Activated TGF-β can bind to TGFBR1/2, which initiates canonical SMAD pathways by phosphorylating SMAD2/3 (15,16). Then, SMAD4 is recruited by phosphorylated-SMAD2/3 and translocates to the nucleus to activate TGF-β-responsive target genes (17). SMAD1/5/9 are targets of the bone morphogenetic protein (BMP) receptor, regarded as non-canonical SMAD-independent pathways (18). SMAD4 coupling with phosphorylated SMAD1/5/9 can mediate antitumour functions (19). Typically, TGF-β inhibits cell proliferation by arresting cells in the G1 phase through increasing the expression of p15 and p21 (20). In cancer cells, the overexpression or genomic mutations of oncogenes reversed the functions of TGF-β by downregulating p15 and p21 (21). TGF-β signalling participated in tumour angiogenesis by inducing a proangiogenic environment (22).
TGF-β is one of the major regulators of extracellular matrix proteins in vascular cells (23). TGF-β1 has been shown to enhance angiogenesis by upregulating VEGF and recruiting more ECs (24). However, the inhibited effects of TGF-β signalling on cancer cells did not match the role of pro-angiogenesis in the early stage of tumourigenesis. We thus deduced that TGF-β signalling would exhibit dual roles in orchestrating tumour angiogenesis. Here, we attempted to analyse the relationships between MVD and TGF-β signalling. By mfIHC staining, we established a dataset of eight quantitatively elevated TGF-β signalling proteins (TGFB1, TGFBR1, TGFBR2, SMAD4, SMAD2/3, p-SMAD2/3, SMAD1/5/9, and p-SMAD1/5/9) in the tissues of CRC patients (9). To address the relevance of tumour vessels and TGF-β signalling proteins, we further stained CD31 to represent the MVD of each sample using the same method and inferred the clines of the dual roles of TGF-β signalling. In addition, we confirmed that the activation of TGF-β signalling could promote tumour angiogenesis in cancer cells, while the excessive activation of TGF-β signalling could prevent tumour angiogenesis.
Methods
Patients
The HCol-Ade180Sur-07 tumour tissue microarray (Shanghai OUTDO Biotech Co., Shanghai, China) consisted of 90 paired colorectal adenocarcinoma tissues and matched normal mucosa (25). Patients underwent surgery from January 2009 to October 2009, and follow-up information was available from February 2009 to May 2014. All sample donors provided informed consent, and the study was conducted under the approval of the Institutional Ethics Committee of Beijing Chaoyang Hospital of Capital Medical University. All tissue samples were collected from patients with colon cancer for whom chemotherapy or radiotherapy was not received before surgery. All procedures were performed in accordance with the relevant guidelines and regulations. All CRC patients were recruited based on the histopathology results. Tumour stage was determined according to the National Comprehensive Cancer Network (NCCN) criteria.
Immunostaining of TMA
The TMA slides were stained using the PerkinElmer OpalTM 4-colour fIHC Kit (Cat#, NEL80001KT, PerkinElmer, Hopkinton, MA, USA). Briefly, the deparaffinization protocol was run (xylene 15 min two times, 100%, 95%, 85%, and 75% ethanol for 10 min) until rinsing in water for 10 min. Then, the slides were pretreated with antigen retrieval solution (provided in the Opal kit) by microwaving method (45 seconds on 100% power, followed by 15 min on 20% power), followed by 10 min blocking in 10% goat serum in TBST and rinsing in water. Then, the TMA slides were stained with a primary antibody against CD31 (dilution, 1:1,000, Cat#: ZA0568, Zhongshan Golden Bridge, Beijing, China) for 1 hour at room temperature. After washing the slides three times with 1X TBST, the slides were incubated with horseradish peroxidase-labelled rabbit secondary antibody (Cat#: SAP9001, Zhongshan Golden Bridge, Beijing, China) for 10 min. A tyramide-conjugated fluorophore (Opal520) was added to the slides at a 1:100 dilution in Amplification Plus Buffer and incubated for 10 min at RT. The conjugated antibody was removed by microwave. The TMA slides were further stained with a primary antibody against SMAD4 (dilution 1:2,000, Cat#: sc-7966, Santa Cruz) for 1 hour at room temperature. After washing with 1X TBST, the slide was subjected to labelling with a tyramide-conjugated fluorophore (Opal620), and the microwave removed the conjugated antibody. Finally, the slide was counterstained with Spectral DAPI to show nuclei and cover slips, followed by sealing with mounting medium supplied with antifade solution (Applygen Technologies Inc. Beijing, China).
Quantitatively calculated MVD by multispectral images
InForm 2.1.1 software (Perkin Elmer) was used for the batch analysis of multispectral images from the experiment. The images were loaded into InForm to build a single colour compensation library and substrate background using the image derived from a slide without staining in the same exposure settings. To calculate the number of MVD, three to six representative regions of interest for high-powered (200×) imaging from every case were selected. To build algorithms for segment tissues and cells, a few representative multispectral images from the experiment were loaded into InForm software. Then, the segmented tissues of parenchymal neoplasms and mesenchyme and cells were trained according to the 4',6-diamidino-2-phenylindole (DAPI) signal intensity. Next, the detected tissue compartments were selected and quantified for each stained protein on the slides. InForm software was used to quantitatively measure the number of CD31-stained MVD. All remaining multispectral images from the experiment were batch processed.
Western blotting
In all, 30 µg of cell lysates was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA). The membranes were blocked with 5% milk and then incubated with various primary antibodies at 4 °C overnight. The antibodies were directed against the following: VEGFA (dilution 1:500, Cat#: ab46154, Abcam, Cambridge, MA, USA), TGFB1 (1:100, Cat#: 3711, CST, Danvers, MA, USA), SMAD4 (1:500, Cat#: sc-7966, Santa Cruz, CA, USA), TGFBR1 (1:200, Cat#: ab135814, Abcam), and TGFBR2 (1:400, Cat#: ab78419, Abcam). Then, the membranes were incubated with the IRDye 680RD goat anti-mouse/rabbit IgG secondary antibodies at 1:10,000 dilutions (Cat#:926-68070/68071, LI-COR, NE, USA).
HUVEC Matrigel tube-forming assay
Initially, Matrigel (Cat#:356234, BD Biosciences) was polymerized (200 µL/well of a 48-well culture plate) for 30 min at 37 °C. Serum-starved HUVECs were resuspended in endothelial cell medium (ECM) media and reseeded onto Matrigel-coated wells (105 cells/well). The human TGFB1 protein [Cat#: 5154, cell signaling technology (CST)] was incorporated into the ECM and cultured with HUVECs. After 24 hours, images of capillary-like networks were recorded using a phase-contrast microscope. The images were analysed with the Angiogenesis Analyzer plugin for ImageJ (Carpentier et al., Angiogenesis Analyzer for ImageJ. 4th ImageJ User and Developer Conference Proceedings).
TCGA dataset analysis and statistics
Molecular data from human colon cancers (COAD) were generated by the TCGA Research Network (http://www.cbioportal.org/). The significance of the data from patient specimens was determined by the Mann-Whitney U test. Overall survival (OS) rates were assessed by the Kaplan-Meier test, and the log-rank test was used to plot survival curves. P<0.05 was considered significant.
Results
mfIHC stains CD31 in the tissues of CRC
mfIHC was used to stain CD31 in 90 paired CRC tissues (cancer vs. adjacent normal tissues). We explored a scoring system based on InForm software to quantitatively evaluate CD31-stained MVD in CRC cancer tissues. As expected, the MVD was significantly higher in cancer tissues (means ± SD, 32.96±26.01) than in normal tissues (13.40±19.45) (Figure 1A). Tumour microvasculature is one of the most important components of the tumour microenvironment. mfIHC showed the microvasculature located in the stroma of cancerous tissues (Figure 1B). In comparison, SMAD4-stained cancer cells were previously demonstrated to be expressed by cancer cells (9). Our results also demonstrated that high MVD (n=47) for CRC patients predicted a shorter OS time than those patients with low MVD (n=39) (Figure 1C).
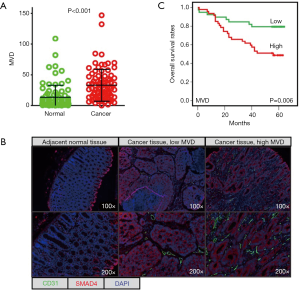
MVD associates with advanced clinical pathological features
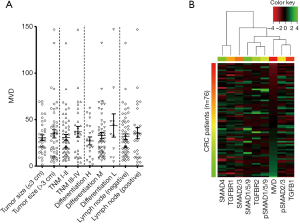
Next, we analysed the relationships between MVD and clinical pathological characteristics. The CRC patients were characterized by tumour size (≤3 vs. >3 cm), TNM stage (I–II vs. III–IV), differentiation (high vs. moderate vs. low), and lymph node status (negative vs. positive) (Figure 2A). Although there were no statistically significant differences, CRC patients with a higher degree of malignancy (tumour size >3 cm, TNM III–IV, poor differentiation, positive lymph node) were found to have higher MVD.
mfIHC analysis reveals the dual effects of TGF-β signalling on regulating angiogenesis
Unsupervised hierarchical clustering was used to explore the relationships between MVD and TGF-β signalling (Figure 2B). The MVD was ordered from low to high and clustered with pSMAD2/3 and TGFB1 into a subgroup. However, this analysis did not successfully divide TGF-β signalling into significantly expressed clusters. Generally, TGFB1 and pSMAD2/3 were positively associated with MVD in patients with low MVD. However, TGFB1 and pSMAD2/3 were negatively associated with MVD in patients with high MVD. Based on these findings, we speculated that TGF-β signalling also exhibited dual effects on orchestrating tumour angiogenesis in CRC.
A low dose of TGFB1 increases VEGFA expression but a high dose of TGFB1 inhibits VEGFA expression
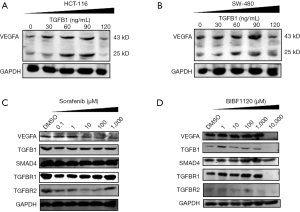
VEGFA is recognized as the most important angiogenetic cytokine and serves as a target of anti-tumour agents, such as sorafenib. To determine the effects of TGF-β signalling on the expression of VEGFA, we used human recombinant TGFB1 proteins to treat HCT116 and SW480 cells. In both HCT116 and SW480 cells, TGFB1 increased the expression of VEGFA when used at a relatively low dose (30~90 ng/mL) (Figure 3A). However, when 120 ng/mL TGFB1 was used to treat HCT116 and SW480 cells, the VEGFA levels decreased (Figure 3B).
Angiogenetic inhibitors either promote or inhibit TGF-β signalling
The strategy of targeting the VEGFA pathway to inhibit tumour progression has been widely used in clinical practice for the treatment of advanced CRC patients. However, the effectiveness of these drugs was limited. We speculated that the dual roles of TGF-β signalling might contribute to changes in the anti-angiogenetic effects of those drugs. To verify this hypothesis, we detected VEGFA and proteins involved in TGF-β signalling after stimulating HCT116 cells with a series of concentrations of sorafenib for 24 hours. The levels of VEGFA were decreased in a dose-dependent manner (Figure 3C). TGFB1, TGFBR1, and TGFBR2 were decreased when treated with 0.1 µM sorafenib. Subsequently, TGFB1 and TGFBR1 were increased after treatment with 1, 10, 100, and 1,000 µM sorafenib. TGFBR2 was increased under stimulation with 1,000 µM sorafenib. In addition, BIBF1120 is an inhibitor of VEGFR and was administered to HCT116 cells at various concentrations (Figure 3D). BIBF1120 did not change the levels of VEGFA when the concentrations were less than 1,000 µM. However, a high dose of BIBF1120 (10,000 µM) deleted the expression of VEGFA, TGFB1, TGFBR1, and TGFBR2. Moreover, low-dose BIBF1120 (<1,000 µM) increased the levels of TGFB1 and TGFBR1. Both sorafenib and BIBF1120 failed to change the levels of SMAD4.
Excessive TGFB1 inhibited HUVECs to form tubes-like structures
Next, HUVEC Matrigel tube-forming assays were applied to determine the effects of TGFB1 on angiogenesis. HUVECs were seeded onto Matrigel-coated wells with media containing human recombinant TGFB1 proteins. The media with 30 ng/mL TGFB1 increased HUVECs to form tube-like structures when compared with the treatment in which HUVECs were cultured with media without TGFB1. However, media with 300 ng/mL TGFB1 did not enhance the ability of HUVECs to form tubes (Figure 4A). The number of branches and total length of the HUVEC-forming tube were calculated after treatment with media supplied with none, low-, or high-dose TGFB1 (Figure 4 B,C). The branches and lengths were significantly increased by treatment with low-dose TGFB1 but were decreased by treatment with high-dose TGFB1.
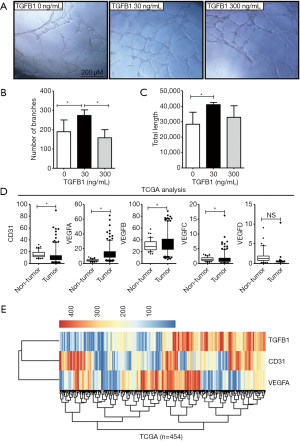
Next, we analysed the differential expression of genes in the TCGA COAD datasets, which contained the normalized values of colon cancer tissues by RNA-sequencing. Here, we compared the mRNA levels of CD31 and the VEGF family (including VEGFA, VEGFB, VEGFC, and VEGFD) between tumour tissues (n=454) and non-tumour tissues (n=41) (Figure 4D). CD31, VEGFA, VEGFB, and VEGFC showed significantly higher expression in tumour tissues than in non-tumour tissues (P<0.05). Unsupervised hierarchical clustering was further used to analyse the expression profiles of the mRNA levels of CD31, VEGFA, and TGFB1 (Figure 4E). These data did not reveal significant correlations in expression. In detail, CRC patients with high TGFB1 levels included patients with either high or low CD31 or VEGFA levels, and vice versa. The profiles of CD31 and TGFB1 were similar, and mfIHC identified patterns for CD31 and TGFB1 on protein levels. The profiles of VEGFA and CD31 also failed to cluster the significantly positive or negative correlation patterns on mRNA levels. The above data partially supported our conclusion that low activated TGF-β signalling enhanced tumour angiogenesis, but excessive TGF-β signalling abolished tumour angiogenesis.
Discussion
Both our previous study (9) and another study (26) revealed hyperactive TGF-β signalling in CRC. The mfIHC allowed us to quantitatively analyse the relationships between TGF-β signalling and tumour angiogenesis in CRC tissues. The present study provides an opportunity to consider the novel application for proteins involving TGF-β signalling that are used as biomarkers to conduct antiangiogenic therapy.
As noted, TGF-β signalling plays contradictory roles in cancer and has been extensively explored by many studies (16,27). This paradox is reflected in the clinic, where in early stage cancers, the levels of TGF-β are positively associated with a favourable prognosis. However, in advanced tumours, the levels of TGF-β are positively associated with tumour size, invasiveness, and dedifferentiation (28). However, few studies have reported the contradictory roles of TGF-β signalling in orchestrating tumour angiogenesis. Normally, angiogenesis is tightly controlled by a balance of angiogenic and antiangiogenic factors. VEGF is recognized as the most critical and specific factor that stimulates both physiological and pathological angiogenesis (29). TGF-β expression was positively and substantially associated with VEGF expression and angiogenesis in late-stage CRC (30). Tumour-derived TGF-β was proven to regulate the proliferation and migration of vascular ECs (31). TGF-β was proven to bind ALK5 and ACVRL1, both of which exhibited antagonistic effects (32). Furthermore, at high concentrations of TGF-β, the canonical SMAD2/3 pathway is activated by ALK5, inducing the expression of plasminogen activator inhibitor and fibronectin, thereby impeding angiogenesis (33). At low concentrations, TGF-β promoted EC cell proliferation and migration by enhancing the expression of MMPs through ALK1 activation of SMAD1/5 (34). In the present study, mfIHC staining supported that TGF-β signalling showed positive roles at low activity in stimulating angiogenesis and increasing VEGFA expression. However, excessive TGF-β signalling acted as a negative regulator of angiogenesis and decreased VEGFA expression. This phenotype turnover might be the reason that the hyperactivation of TGF-β signalling prolonged the OS time by inhibiting tumour angiogenesis in patients with CRC.
VEGF is a factor of angiogenesis that includes several alterative isoforms with significantly different functions (2). VEGF165 antagonized VEGF165b-induced EC proliferation and competitively bound to VEGFR2 (35). Bevacizumab is a humanized monoclonal antibody against VEGF and has been used for CRC therapy by blocking all angiogenic isoforms of VEGF (36). The polymorphisms of VEGF have been found to be correlated with the efficacy of bevacizumab (37). Moreover, bevacizumab was proven to be less effective in obese metastatic CRC patients (38). The genetic variations of TGFB1 did not find any relationships with the outcomes for CRC patients who received bevacizumab (39). In contrast, our present study provides evidence that the protein levels for those factors involved in TGF-β signalling might contribute to predicting the efficacy of bevacizumab-based chemotherapy.
In conclusion, our study is the first to report the dual roles of TGF-β signalling in orchestrating tumour angiogenesis in CRC. mfIHC provides the ability to simultaneously and quantitatively analyse TGF-β signalling and MVD. The main finding shows that low active TGF-β signalling enhances angiogenesis, but hyperactive TGF-β signalling inhibits angiogenesis. Further mechanistic studies will need to address the hyperactive TGF-β signalling that abolishes angiogenesis and benefits chemotherapy by anti-angiogenic drugs.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.02.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All sample donors provided informed consent, and the study was conducted under the approval of the Institutional Ethics Committee of Beijing Chaoyang Hospital of Capital Medical University (No. 2018-ke-24). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Ahluwalia A, Jones MK, Matysiak-Budnik T, et al. VEGF and colon cancer growth beyond angiogenesis: does VEGF directly mediate colon cancer growth via a non-angiogenic mechanism? Curr Pharm Des 2014;20:1041-4. [Crossref] [PubMed]
- Chabowski M, Nowak A, Grzegrzolka J, et al. Comparison of Microvessel Density Using Nestin and CD34 in Colorectal Cancer. Anticancer Res 2018;38:3889-95. [Crossref] [PubMed]
- Rong W, Yang L, Yin L, et al. PSG9 promotes angiogenesis by stimulating VEGFA production and is associated with poor prognosis in hepatocellular carcinoma. Sci China Life Sci 2017;60:528-35. [Crossref] [PubMed]
- Yang L, Hu S, Tan J, et al. Pregnancy-specific glycoprotein 9 (PSG9), a driver for colorectal cancer, enhances angiogenesis via activation of SMAD4. Oncotarget 2016;7:61562-74. [PubMed]
- Li H, Yu L, Wang W, et al. Dynamics of angiogenesis and cellularity in rabbit VX2 tumors using contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging. Oncol Lett 2018;15:2978-84. [PubMed]
- Huang J, Ma X, Chen X, et al. Microvessel density as a prognostic factor in bladder cancer: a systematic review of literature and meta-analysis. Cancer Biomark 2014;14:505-14. [Crossref] [PubMed]
- Mitrofanova I, Zavyalova M, Riabov V, et al. The effect of neoadjuvant chemotherapy on the correlation of tumor-associated macrophages with CD31 and LYVE-1. Immunobiology 2018;223:449-59. [Crossref] [PubMed]
- Yang L, Liu Z, Tan J, et al. Multispectral imaging reveals hyper active TGF-beta signaling in colorectal cancer. Cancer Biol Ther 2018;19:105-12. [Crossref] [PubMed]
- Muppala S, Xiao R, Krukovets I, et al. Thrombospondin-4 mediates TGF-beta-induced angiogenesis. Oncogene 2017;36:5189-98. [Crossref] [PubMed]
- Katz LH, Likhter M, Jogunoori W, et al. TGF-beta signaling in liver and gastrointestinal cancers. Cancer Lett 2016;379:166-72. [Crossref] [PubMed]
- Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 2015;160:963-76. [Crossref] [PubMed]
- Chen J, Katz LH, Munoz NM, et al. Vitamin D Deficiency Promotes Liver Tumor Growth in Transforming Growth Factor-beta/Smad3-Deficient Mice Through Wnt and Toll-like Receptor 7 Pathway Modulation. Sci Rep 2016;6:30217. [Crossref] [PubMed]
- Yin S, Fan Y, Zhang H, et al. Differential TGFbeta pathway targeting by miR-122 in humans and mice affects liver cancer metastasis. Nat Commun 2016;7:11012. [Crossref] [PubMed]
- Costanza B, Umelo IA, Bellier J, et al. Stromal Modulators of TGF-beta in Cancer. J Clin Med 2017;6. [PubMed]
- Dews M, Tan GS, Hultine S, et al. Masking epistasis between MYC and TGF-beta pathways in antiangiogenesis-mediated colon cancer suppression. J Natl Cancer Inst 2014;106:dju043. [Crossref] [PubMed]
- Mullen AC, Orlando DA, Newman JJ, et al. Master transcription factors determine cell-type-specific responses to TGF-beta signaling. Cell 2011;147:565-76. [Crossref] [PubMed]
- Zhang YH, Cheng F, Du XT, et al. GDF11/BMP11 activates both smad1/5/8 and smad2/3 signals but shows no significant effect on proliferation and migration of human umbilical vein endothelial cells. Oncotarget 2016;7:12063-74. [PubMed]
- Hernanda PY, Chen K, Das AM, et al. SMAD4 exerts a tumor-promoting role in hepatocellular carcinoma. Oncogene 2015;34:5055-68. [Crossref] [PubMed]
- Haque S, Morris JC. Transforming growth factor-beta: A therapeutic target for cancer. Hum Vaccin Immunother 2017;13:1741-50. [Crossref] [PubMed]
- Zheng L, Suzuki H, Nakajo Y, et al. Regulation of c-MYC transcriptional activity by transforming growth factor-beta 1-stimulated clone 22. Cancer Sci 2018;109:395-402. [Crossref] [PubMed]
- Jiang X, Shan J, Dai N, et al. Apurinic/apyrimidinic endonuclease 1 regulates angiogenesis in a transforming growth factor beta-dependent manner in human osteosarcoma. Cancer Sci 2015;106:1394-401. [Crossref] [PubMed]
- Boguslawska J, Rodzik K, Poplawski P, et al. TGF-beta1 targets a microRNA network that regulates cellular adhesion and migration in renal cancer. Cancer Lett 2018;412:155-69. [Crossref] [PubMed]
- Petersen M, Pardali E, van der Horst G, et al. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 2010;29:1351-61. [Crossref] [PubMed]
- Lu Y, Zhao X, Liu Q, et al. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/beta-catenin signaling. Nat Med 2017;23:1331-41. [Crossref] [PubMed]
- Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, et al. Prognostic significance of transforming growth factor beta (TGF-beta) signaling axis molecules and E-cadherin in colorectal cancer. Tumour Biol 2012;33:1005-14. [Crossref] [PubMed]
- Chun HK, Jung KU, Choi YL, et al. Low expression of transforming growth factor beta-1 in cancer tissue predicts a poor prognosis for patients with stage III rectal cancers. Oncology 2014;86:159-69. [Crossref] [PubMed]
- Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-beta-induced EMT during cancer progression. Cell Tissue Res 2012;347:85-101. [Crossref] [PubMed]
- Lan J, Li H, Luo X, et al. BRG1 promotes VEGF-A expression and angiogenesis in human colorectal cancer cells. Exp Cell Res 2017;360:236-42. [Crossref] [PubMed]
- Xiong B, Gong LL, Zhang F, et al. TGF beta1 expression and angiogenesis in colorectal cancer tissue. World J Gastroenterol 2002;8:496-8. [Crossref] [PubMed]
- Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med 2003;13:301-7. [Crossref] [PubMed]
- Aspalter IM, Gordon E, Dubrac A, et al. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun 2015;6:7264. [Crossref] [PubMed]
- Goumans MJ, Valdimarsdottir G, Itoh S, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell 2003;12:817-28. [Crossref] [PubMed]
- Principe DR, Doll JA, Bauer J, et al. TGF-beta: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst 2014;106:djt369. [Crossref] [PubMed]
- Nagasaki M, Kondo S, Mukudai Y, et al. Clinicopathological implications of vascular endothelial growth factor 165b expression in oral squamous cell carcinoma stroma. Oncol Rep 2016;36:573-81. [Crossref] [PubMed]
- Yamashita-Kashima Y, Fujimoto-Ouchi K, Yorozu K, et al. Biomarkers for antitumor activity of bevacizumab in gastric cancer models. BMC Cancer 2012;12:37. [Crossref] [PubMed]
- Caulet M, Lecomte T, Bouche O, et al. Bevacizumab Pharmacokinetics Influence Overall and Progression-Free Survival in Metastatic Colorectal Cancer Patients. Clin Pharmacokinet 2016;55:1381-94. [Crossref] [PubMed]
- Artac M, Korkmaz L, Coskun HS, et al. Bevacuzimab May Be Less Effective in Obese Metastatic Colorectal Cancer Patients. J Gastrointest Cancer 2018; [Epub ahead of print]. [PubMed]
- Stremitzer S, Zhang W, Yang D, et al. Genetic variations in angiopoietin and pericyte pathways and clinical outcome in patients with resected colorectal liver metastases. Cancer 2015;121:1898-905. [Crossref] [PubMed]


