
The feasibility and safety of modified robot-assisted enucleation for highly complex renal tumors: research on a surgical technique
Introduction
During the past decades, the standard treatment of localized renal tumors has been nephron-sparing surgery (NSS) via laparoscopy or a robot-assisted laparoscopy approach (1,2). Whenever it is technically practicable, NSS has been encouraged for the treatment of larger (≥4 cm) renal tumor (3,4). Nevertheless, there are few reports which discuss the removal of the renal tumors, particularly those with high surgical complexity, or how to reduce postoperative complications and prevent tumor recurrence for the treatment of highly complex renal tumors. In order to achieve these goals, we developed a modified robot-assisted tumor enucleation (MRATE) technique, and present them in this article.
Methods
Patients
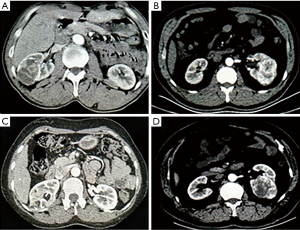
From September 2014 to August 2017, a total of 175 patients with highly complex renal tumors who were treated with conventional robot-assisted partial nephrectomy (C-RAPN) or MRATE by a single surgeon. Nine patients were excluded based on the following exclusion criteria: patients with multiple renal neoplasms, metastatic renal cell carcinoma (RCC), and patients who underwent a second procedure together with NSS. The remaining 166 cases were divided into two groups: 94 patients were treated with MRATE, and Seventy-two patients who underwent C-RAPN. The decision for the surgical approach (i.e., C-RAPN, MRATE) was made based on preoperative tumor characteristics and cross-sectional imaging. Specifically, tumors with a distinct pseudocapsule, which was displayed by imaging enhancement or ultrasonic contrast, were commonly treated with MRATE (Figure 1A,B,C,D).
Data collection
Preoperative clinical information included age, gender, body mass index (BMI), tumor size, estimated glomerular filtration rate (eGFR) level, and Charlson score. The Charlson comorbidity index (CCI) system was used to score patients according to no major physical comorbidity (CCI =0) or any major physical comorbidity (CCI ≥1). Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) were used to evaluate the surgical complexity of tumor by two urology experts and a radiologist using the PADUA classification system. High surgical complexity was defined by a PADUA score ≥10 (Figure 1A,B,C,D) (5).
Perioperative outcomes included total operation time (TOT), estimated blood loss (EBL), warm ischemia time (WIT), and postoperative hospitalization. A single genitourinary pathologist evaluated postoperative pathological variables, which included the average thickness of pseudocapsule (PC), histological subtypes, nuclear grade, average margin width, along with cancer-parenchyma interface (CPI) width and surgical margin. Average margin width was defined as the distance from the incisive margin to tumor PC. CPI was defined as the distance from the normal parenchymal margin to the tumor PC. eGFR was measured at postoperative day 1, month 3 and month 6 using the modification of diet in renal disease formula (6). Postoperative complications were ranked according to the Clavien-Dindo classification (7).
Surgical technique
The technique of MRATE is based on pathologic and anatomic bases: (I) The vast majority of malignant renal tumor form a distinct fibrous PC, which is mainly composed of fibrous tissue and compressed renal parenchyma (8); (II) The CPI is histologically altered with evidence of inflammation, glomerulosclerosis (GS), nephrosclerosis (NS) and arteriosclerosis (AS) adjacent to the tumor PC, and degree of inflammation, GS, NS and AS relieve with increasing distance from tumor PC (8); (III) In addition, the composition of the CPI varies in different areas of the same tumor. For instance, if the bases of the tumor are close to renal sinus or collection system, cancer-parenchyma interface may disappear (8). (IV) Intrarenal vessels in this pathological change area immediately adjacent to the PC are generally smaller in caliber and fewer in number (8).
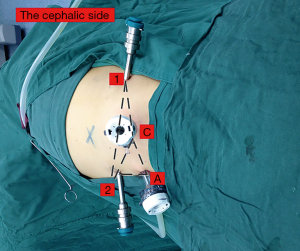
The combined retroperitoneoscopic and transperitoneoscopic accesses are shown in Figure 2 (9). Patients were placed in the lateral position at approximately 90°. After a 2-cm incision was made at the mid-iliac crest, the finger tip mobilized the peritoneum, and then the retroperitoneal space was expanded using a balloon dilator. The 4-trocar robotic-assisted laparoscopic approach was used. A standard 12-mm camera trocar was placed at the first incision, one 8-mm robotic trocar was placed at the lateral border of the paraspinal muscles, and one 10-mm assistant trocar was placed approximately 8cm from the iliac crest at the anterior axillary lines. Subsequently, the peritoneum was opened, and another 8-mm robotic trocar was placed at the peritoneum stomata. The MRATE procedure involved the use of the da Vinci Si robot (Intuitive Surgical, CA, USA) and is displayed in Figure 3.
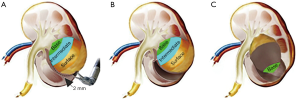
After a bulldog clamp controlled the renal artery, the perirenal fat directly above the tumor was retracted for tumor lifting. The enucleation plane was used for keeping a 2-mm sliver of parenchymal tissue on the superficial and the intermediate surface of the tumor (Figure 3A,B) rather than completely along the tumor PC. When the natural cleavage plane was closer to the base of the tumor, the tumor was gently yet tautly elevated from the tumor bed with blunt dissection by placing the robotic bipolar forceps into the enucleation plane (Figure 3C), so the tumor could be easily lifted away from the kidney. When reaching the renal sinus, if the larger renal artery directly branched into the tumor, the tumor would be controlled with hemlock clips. Renal calyceal system entry was fixed (4-0 Vicryl on an SH-1 needle), and two hemlocks were placed in each passage to apply enough tension to the stitch. It is recommended to suture the renal parenchyma defect in 2 layers due to the complexity of the tumor. For C-RAPN, the tumor PC was not visually identified, and an additional 5–10 mm of renal parenchyma around the tumor PC was resected during operation.
Follow-up
Patients were generally followed up with every three months for the first year after NSS and every six months after that. Follow-up consisted of a physical examination, routine blood analysis, and radiographic evaluation of the kidneys and chest. Cause-specific survival rates of MRATE and C-RAPN were assessed through a review of hospital medical records or contact with patients.
Statistical analysis
All statistical analyses were performed using the SPSS (ver. 20 software package). The Student’s t-test and the one-way ANOVA test was used to assess continuous variables. The Pearson chi-square test or Fisher’s exact tests was used for evaluating the categorical variables. The Kaplan-Meier method was used to calculate the cause-specific survival rate. A two-sided P<0.05 was considered statistically significant.
Results
The demographics of 166 cases of renal tumors characteristics are summarized in Table 1. Seventy-two patients who underwent C-RAPN, and 94 patients were treated with MRATE. Overall, the MRATE and C-RAPN groups presented similar age, sex, BMI, Charlson score, along with mean tumor size and PAUDA score. Perioperative outcomes of 166 cases of renal tumors are described in Table 2. Compared with C-RAPN group, MRATE group showed less EBL (90.76 vs. 143.29 mL, P<0.001), shorter postoperative hospitalization (5.99 vs. 6.80 days, P<0.001), lower WIT (17.46 vs. 20.47 min, P<0.001) and shorter TOT (107.14 vs. 121.32 min, P<0.001). Mean reduction in eGFR level at postoperative day 1 was significantly lower in the MRATE group (−9.43 and −12.65 mL/min/1.73 m2, respectively; P<0.001). The resection was carried into the collecting system in 3.2% of the MRATE group compared with 12.5% of the C-RAPN group (P=0.032). Postoperative complication rates were distinctly lower in the MRATE group (4.2% and 16.7%, respectively; P=0.033). Median follow-up of the patients undergoing MRATE and C-RAPN was 36 and 37 months, respectively. Three cases of renal recurrence (distant from the surgical margin) and two cases of the metastatic disease were detected during follow-up in patients with negative margins. Subsequently, three cases of renal recurrence were treated with RN, and no tumor metastasis with follow-up period was detected.
Table 1
| Variable | C-RAPN | MRATE | P |
|---|---|---|---|
| Total patients | 72 | 94 | |
| Age, year, mean ± SD | 52.35±13.46 | 51.37±13.38 | 0.643 |
| Male patients, n (%) | 43 (59.7) | 62 (66.0) | 0.409 |
| Charlson score, mean ± SD | 1.28±1.48 | 1.52±1.50 | 0.299 |
| BMI (kg/m2), mean ± SD | 25.69±3.05 | 24.77±3.33 | 0.071 |
| Tumor size (cm), mean ± SD | 4.77±1.03 | 4.66±1.14 | 0.553 |
| PDUDA score | 0.912 | ||
| 10 | 53 | 67 | |
| 11 | 8 | 14 | |
| 12 | 6 | 7 | |
| 13 | 5 | 6 |
BMI, body mass index.
Table 2
| Variable | C-RAPN | MRATE | P |
|---|---|---|---|
| TOT (min), mean ± SD | 121.32±11.48 | 107.14±10.35 | <0.001 |
| Postoperative hospitalization (day), mean ± SD | 6.80±1.02 | 5.99±0.67 | <0.001 |
| Mean WIT (min), mean ± SD | 20.47±3.46 | 17.46±3.69 | <0.001 |
| EBL (mL), mean ± SD | 143.29±118.26 | 90.76±28.31 | <0.001 |
| Mean preoperative eGFR (mL/min/1.73 m2), mean ± SD | 99.42±18.51 | 107.66±14.20 | 0.002 |
| eGFR reduction from preoperative: (mL/min/1.73 m2), mean ± SD | |||
| Postoperative day 1 | −12.65±5.67 | −9.43±4.09 | <0.001 |
| Postoperative month 3 | −6.25±2.97 | −5.24±2.01 | 0.010 |
| Postoperative month 6 | −3.75±1.68 | −3.48±1.16 | 0.238 |
| Collecting system entry/repair, n (%) | 9 (12.5) | 3 (3.2) | 0.032 |
| Postoperative complication, n (%) | 12 (16.7) | 4 (4.3) | 0.033 |
| Clavein-Dindo grade 1, n (%) | |||
| Haematuria | 2 (2.8) | 2 (2.1) | |
| Clavein-Dindo grade 2, n (%) | |||
| Mild fever | 5 (6.9) | 2 (2.1) | |
| Transfusion | 3 (4.2) | 0 | |
| Clavein-Dindo grade 3, n (%) | |||
| Urinoma | 2 (2.8) | 0 | |
| Median follow-up months [range] | 37 [19–51] | 36 [16–51] | |
| Renal recurrence, n (%) | 1 (1.4) | 2 (2.1) | 0.664 |
| Systemic recurrence, n (%) | 1 (1.4) | 1 (1.1) | 0.960 |
| Cause-specific survival rate (%) | 98.6 | 98.9 | 0.960 |
TOT, total operation time; WIT, warm ischemia time; EBL, estimated blood loss; eGFR, estimated glomerular filtration rate.
Cause-specific survival rates were 98.9% in the MRATE group and 98.6% after C-RAPN, due to two cases of postoperative patients dying of the metastatic disease, 17 and 19 months later, respectively.
Postoperative pathological outcomes of 166 cases of renal tumors are shown in the Table 3. No statistical difference with Fuhrman grade, tumor subtype and positive margins between groups was found. However, the C-RAPN group displayed a larger margin distance compared with the MRATE group (mean, 4.56 vs. 1.82 mm; P<0.001). Two patients diagnosed with positive surgical margins immediately converted to radical nephrectomy (RN), and no tumor recurrence was found during the follow-up period.
Table 3
| Variable | C-RAPN | MRATE | P |
|---|---|---|---|
| Fuhrman grade | 0.279 | ||
| Fuhrman 1 | 21 | 23 | |
| Fuhrman 2 | 43 | 52 | |
| Fuhrman 3–4 | 8 | 19 | |
| Tumor subtype, n (%) | 0.497 | ||
| ccRCC | 58 (80.6) | 70 (74.5) | |
| PRCC | 7 (9.7) | 9 (9.6) | |
| ChRCC | 4 (5.6) | 5 (5.3) | |
| CRCC | 3 (4.2) | 10 (10.6) | |
| Average margin width (mm), mean ± SD | 4.57±0.74 | 1.82±0.24 | <0.001 |
| Specimen with positive margins, n (%) | 1 (1.4) | 1 (1.1) | 1.000 |
ccRCC, clear cell renal cell carcinoma; PRCC, papillary renal cell carcinoma; ChRCC, chromophobe renal cell carcinoma; CRCC, cystic renal cell carcinoma.
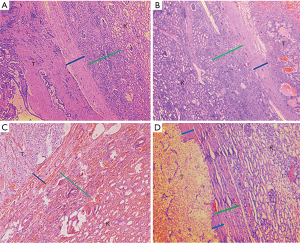
After obtaining institutional review board (IRB) approval (Quick-PJ 2018-07-06), we examined the records of 240 cases of stage pT1b tumors with PADUA scores of ≥10 resected with RN (From 2005 to 2014). The following 15 cases were excluded, and preoperative imaging examination showed that three cases had adrenal metastasis, two cases had renal pelvis invasion; four cases had renal hilar lymph node metastasis, and the pathological report proved six cases with perirenal fat invasion. Pathological characteristics of the remaining 225 cases are reported in Table 4. All data consented to inclusion in our IRB-approved prospective institutional kidney cancer database and an uropathologist examined all archived slides. The histological features of the PC (thickness and invasion), CPI and extra-pseudocapsular extension rate (EPE) were documented. Histological subtypes were divided into three groups: clear cell renal cell carcinoma (ccRCC) (115 cases), papillary renal cell carcinoma (PRCC) (60 cases), and chromophobe renal cell carcinoma (ChRCC) (50 cases). Mean tumor size was 5.30, 5.10, and 4.93 cm for the ccRCC group, PRCC group and ChRCC group, respectively. The average width of PC was 0.60, 0.51 and 0.54 mm in three groups, respectively. Correspondingly, the average CPI was 1.81, 1.62, and 1.89 mm in the three groups. PC invasion was detected in 26.5% of the ccRCC group, 23.6% of the PRCC group and 28.2% of the ChRCC group. EPE was observed in the three groups (3.5%, 3.3%, and 4.0%, respectively). Pathological sections of 225 cases of stage pT1b renal tumors with PADUA scores of ≥10 are displayed in Figure 4. We observed tumor PC (blue line) and CPI (green line) in three subtypes (Figure 4A,B,C). Eight cases of tumor cell infiltrated beyond tumor PC but within CPI (Figure 4D).
Table 4
| Tumor subtype | Number | Tumor size (cm) | Average width of PC (mm) | Average CPI (mm) | PC invasion, (%) | EPE, (%) |
|---|---|---|---|---|---|---|
| ccRcc | 115 | 5.30±1.17 | 0.60±0.16 | 1.81±0.25 | 26.5% | 3.5% |
| PRCC | 60 | 5.10±0.90 | 0.51±0.12 | 1.62±0.12 | 23.6% | 3.3% |
| ChRCC | 50 | 4.93±0.72 | 0.54±0.15 | 1.89±0.23 | 28.2% | 4.0% |
| Total | 225 |
ccRCC, clear cell renal cell carcinoma; PRCC, papillary renal cell carcinoma; ChRCC, chromophobe renal cell carcinoma; CPI, cancer-parenchyma interface; PC, pesudocapsule; EPE, extra-pseudocapsular extension rate.
Discussion
In the past few decades, PN technique has evolved from open, laparoscopic technique into the C-RAPN approach (10). More recently, tumor enucleation technique combined with robotic surgery might be considered a further evolution of the NSS. It may provide the maximum preservation of the normal renal parenchyma, shorter WIT, lower incidental calyceal tearing or vascular injuries for highly complex renal tumors (11,12). The PADUA score is a simple anatomical system and is an independent predictor of complexity for NSS (13). Compared with renal tumors with a score of 8–9 and 6–7, those with a score of ≥10 obtain a 14.5- and 30.6-times higher risk of overall complication rates, respectively (5). Based on this, we defined localized renal tumors with a PADUA score of ≥10 as tumors with a highly complex challenge in the present study.
In general, the localized renal tumor is treated with NSS via the retroperitoneal or transperitoneal approach. The retroperitoneal approach allows direct access to the renal artery and posterior tumor; nevertheless, it leads to fewer anatomical landmarks and limited retroperitoneal space. The transperitoneal approach provides wider working space, clear anatomical landmarks; but it increases the risk of intraperitoneal organ injury and postoperative adhesion (14). Due to the advantage and pitfalls of the surgical approach, we treated complex renal tumors with MRATE or C-RAPN using combined retroperitoneoscopic and transperitoneoscopic accesses, which offer a direct, rapid approach to the renal artery and renal tumors, and sufficient space during surgery.
The MRATE technique of our medical center is based on an analysis of 225cases of malignant renal tumors with PADUA scores of ≥10 resected with RN. CPI of malignant renal tumors was measured (mean 1.8 mm, range, 1.5–2.1 mm). Therefore, the MRATE technique was described as follows: tumor was enucleated along the natural cleavage plane that kept a 2-mm sliver of parenchymal tissue away from the PC at the superficial surface and the intermediate surface of the tumor, and blunt dissection along the tumor PC was performed at the base of the tumor. Recently, Minervini et al. (15) suggested a surface-intermediate-base (SIB) margin score reporting system for the operative techniques during NSS and divided it into five main categories: pure enucleation, hybrid enucleation, enucleoresection, hybrid enucleoresection, and resection. SIB score system was the first standardized reporting system of excision method during PN based on an analysis of renal parenchymal margin visually scored at the superficial surface, the intermediate surface and the base of the tumor. When the SIB score system was applied, MRATE, and C-RAPN techniques in the present study were classified into hybrid enucleation (SIB score 1+1+0=2), and resection (SIB score 1+2+2=5), respectively (16).
The key point of MRATE technique is to separate a 2-mm sliver of cancer-parenchyma interface away from the superficial surface and the intermediate surface of the tumor PC. Once the cleavage plane is entered, the enucleation step grants substantial help. When the enucleating plane is closer to the base of the tumor, the tumor needs to gently yet tautly lift from the renal PC with blunt dissection, which can significantly reduce the risk of damage to vascular structures or the collecting system. Put differently, MRATE has certain advantages over C-RAPN, which include allowing an obvious anatomical cleavage plane during surgery, maximum preservation of kidney parenchyma with complete removal of the tumor tissue, and significantly poorer functional CPI. Furthermore, MRATE avoids damaging the critical collecting system and major blood vessels that are adjacent to the base of high-complexity renal tumors (17). Owing to these advantages, MRATE technique would particularly benefit large or endophytic masses (18). Sometimes, complex renal tumors are ultimately endophytic masses, and often present thicker PC, which can be identified by the presence of a hyperechoic rim around a tumor with the assistance of a ‘drop-in’ ultrasound probe (19). Another condition is a perihilar tumor, which is particularly well suited for enucleation if a maximal kidney parenchymal needs preservation (20). In this study, MRATE group presented less blood loss (90.76 vs. 143.29 mL, P<0.001), lower postoperative complications (4.2% vs. 16.7%, P=0.033), shorter WIT (17.5 vs. 20.5min, P< 0.001), and better renal function at postoperative day 1 (−9.43 vs. −12.65 mL/min/1.73 m2, P<0.001, respectively) compared with that of the C-RAPN group.
Histopathologic analysis of peritumoral PC and CPI is particularly important for the feasibility and reliability of MRATE technique. Azhar et al. (8) reported that the PC was present in 119 tumors (96%). In malignant tumors, the rate of intra-renal PC invasion was 37.5%, and four tumors invaded through tumor PC into the surrounding kidney parenchyma with a median depth of invasion 1.05 (0.3–1.3) mm. Fifty-three tumors with PC invasion had a negative surgical margin. Similar to the present study, we noticed that the tumor PC was detected in 221 cases (98.2%) of stage T1b renal tumors with PADUA scores of ≥10 (Table 4). In the ccRCC, PRCC, and ChRCC groups, the rate of PC invasion was 26.5%, 23.6%, and 28.2%, respectively. The rate of EPE was 3.5%, 3.3%, and 4.0%, respectively. None of the eight tumors with EPE infiltrated through CPI, which may explain why only one case had positive margin in the MRATE group.
Although the risk of the PC invasion is associated with tumor size and grade, PC penetration does not increase the rates of having a local or systemic recurrence (21,22). In the present study, only two cases of local recurrence and one case of the metastatic disease were observed during follow-up in MRATE group. MRATE technique may provide a clear dissection plane for the surgeon and obviate the need to enter into the tumor. More importantly, cancer-parenchyma interface is a barrier to the spread of cancer cells. Similarly, several recent studies have shown oncological safety and therapeutic efficacy of SE with long-term follow-up (23-25).
There are several limitations to this study. First, it is a retrospective analysis, and selection bias cannot be excluded. Furthermore, few cases were included, and the survival rate was assessed at the relatively short follow-up period. Finally, renal function was assessed by total eGFR due to renal scintigraphies was achieved only in selected cases. Nevertheless, encouraging results include the preservation of maximum renal parenchyma, better perioperative outcomes, and oncological safety.
Conclusions
Overall, the MRATE has been proven to be a feasible technique for the treatment of complex renal tumors. Moreover, it is associated with a low risk of postoperative complications, an excellent outcome of the renal function, and oncological safety.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.04.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all patients. The study was approved by institutional review board (IRB) (Quick-PJ 2018-07-06).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tobis S, Venigalla S, Wu G, et al. Robot-assisted partial nephrectomy: analysis of the first 100 casesfrom a single institution. J Robot Surg 2012;6:139-47. [Crossref] [PubMed]
- Chen L, Wang L, Xu D, et al. Selection of personalized laparoscopic partial nephrectomy based ontumor characteristics: A preliminary study in a single center. Int J Surg 2015;23:46-51. [Crossref] [PubMed]
- Tobis S, Wu G, Golijanin D, et al. Near infrared fluorescence imaging with robotic assisted laparoscopic partial nephrectomy: initial clinical experience for renal cortical tumors. J Urol 2011;186:47-52. [Crossref] [PubMed]
- Wu Z, Wang L. Re: Riccardo Bertolo, Riccardo Autorino, Giuseppe Simone, et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T2 Renal Tumors: A Multicenter Analysis (ROSULA Collaborative Group). Eur Urol 2018;74:226-32. Eur Urol 2018;74:e145-6. [Crossref] [PubMed]
- Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumors in patients who are candidates for nephron-sparing surgery. Eur Urol 2009;56:786-93. [Crossref] [PubMed]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Azhar RA, de Castro Abreu AL, Broxham E, et al. Histological analysis of the kidney tumor-parenchyma interface. J Urol 2015;193:415-22. [Crossref] [PubMed]
- Tai S, Mahamuni S, Liang CZ, et al. Combined Retroperitoneoscopic and Transperitoneoscopic Accesses for Robot-Assisted Partial Nephrectomy. Journal of Endourology and Videourology 2018. Available online: https://doi.org/
10.1089/vid.2017.0060 - Wu Z, Wang L, Sun Y, et al. Propensity-score matched analysis comparing robot-assisted with laparoscopic partial nephrectomy. BJU Int 2015;115:437-45. [Crossref] [PubMed]
- Zhao X, Lu Q, Guo H, et al. Endoscopic Robot-assisted Simple Enucleation Versus Laparoscopic Simple Enucleation With Single-layer Renorrhaphy in Localized Renal Tumors: A Propensity Score-matched Analysis From a High-volume Centre. Urology 2018;121:97-103. [Crossref] [PubMed]
- Serni S, Vittori G, Frizzi J, et al. Simple enucleation for the treatment of highly complex renal tumors: perioperative, functional and oncological results. Eur J Surg Oncol 2015;41:934-40. [Crossref] [PubMed]
- Zhuang J, Lian H, Guo H, et al. The application of PADUA scoring system for predicting complications of laparoscopic renal cryoablation. Int Urol Nephrol 2015;47:781-8. [Crossref] [PubMed]
- Marszalek M, Chromecki T, Al-Ali BM, et al. Laparoscopic partial nephrectomy: a matched-pair comparison of the transperitoneal versus the retroperitoneal approach. Urology 2011;77:109-13. [Crossref] [PubMed]
- Minervini A, Carini M, Uzzo RG, et al. Standardized reporting of resection technique during nephron-sparing surgery: the surface-intermediate-base margin score. Eur Urol 2014;66:803-5. [Crossref] [PubMed]
- Cao D, Bai L, Wei Q, et al. Re: Comparison of Surgical Outcomes Between Resection and Enucleation in Robot-Assisted Laparoscopic Partial Nephrectomy for Renal Tumors According to the Surface-Intermediate-Base Margin Score: A Propensity Score-Matched Study (From: Takagi T, Kondo T, Tachibana H, et al. J Endourol 2017;31:756-761). J Endourol 2018;32:360-1. [Crossref] [PubMed]
- Lu Q, Zhao X, Guo H, et al. Modified laparoscopic simple enucleation with single-layer suture technique versus standard laparoscopic partial nephrectomy for treating localized renal cell carcinoma. Int Urol Nephrol 2017;49:239-45. [Crossref] [PubMed]
- Wang B, Gong H, Zhang X, et al. Retroperitoneal Laparoscopic Nephrotomy Along the Brodel Line and Tumor Enucleation for Complete Intraparenchymal Renal Tumors: A Single Institution Experience. J Endourol 2017;31:1044-8. [Crossref] [PubMed]
- Volpe A, Garrou D, Amparore D, et al. Perioperative and renal functional outcomes of elective robot-assisted partial nephrectomy (RAPN) for renal tumors with high surgical complexity. BJU Int 2014;114:903-9. [Crossref] [PubMed]
- González J, Cózar JM, Gómez A, et al. Nephron-sparing surgery in renal cell carcinoma: current perspectives on technical issues. Curr Urol Rep 2015;16:6. [Crossref] [PubMed]
- Lu Q, Ji C, Guo H, et al. Histopathologic analysis of tumor bed and peritumoral pseudocapsule after in vitro tumor enucleation on radical nephrectomy specimen for clinical T1b renal cell carcinoma. Urol Oncol 2017;35:603.e15-603.e20. [Crossref] [PubMed]
- Minervini A, Di Cristofano C, Lapini A, et al. Histopathologic analysis of peritumoral pseudocapsule and surgical margin status after tumor enucleation for renal cell carcinoma. Eur Urol 2009;55:1410-8. [Crossref] [PubMed]
- Minervini A, Serni S, Tuccio A, et al. Simple enucleation versus radical nephrectomy in the treatment of pT1a and pT1b renal cell carcinoma. Ann Surg Oncol 2012;19:694-700. [Crossref] [PubMed]
- Cao DH, Liu LR, Wei Q, et al. Simple tumor enucleation may not decrease oncologic outcomes for T1 renal cell carcinoma: A systematic review and meta-analysis. Urol Oncol 2017;35:661.e15-661.e21. [Crossref] [PubMed]
- Serni S, Vittori G, Frizzi J, et al. Simple enucleation for the treatment of highly complex renal tumors: perioperative, functional and oncological results. Eur J Surg Oncol 2015;41:934-40. [Crossref] [PubMed]


