
Hepatobiliary case report and literature review of hepatic reactive lymphoid hyperplasia with positive anti-smooth muscle antibody and anti-nuclear antibody tests
Introduction
Reactive lymphoid hyperplasia (RLH) is a benign nodular lesion, clinically recognized as a pseudolymphoma, affecting various organs including the gastrointestinal tract, orbit, lung, skin and thyroid (1). Histopathological characteristics of RLH include marked proliferation of nonneoplastic, polyclonal lymphocytes that form follicles with active germinal centers. Most reported RLH cases are associated with other malignant gastrointestinal tumors, hepatic virus infections or autoimmune diseases. This case report discusses a case of RLH in liver that was characterized by mostly normal laboratory tests except for positive anti-smooth muscle antibody (SMA) and anti-nuclear antibody (ANA) tests and that had no direct complications.
Case presentation
Chief complaints
A 69-year-old female was admitted for further evaluation of an evident hepatic mass discovered one month earlier. The patient initially complained of intermittent abdominal distention without other discomfort.
History of present illness
The patient suffered from intermittent abdominal distention without other discomfort for two months, and CT scan showed an evident hepatic mass discovered one month before she was admitted into hospital.
History of past illness
The patient had a past history of hypertension for 40 years and type II diabetes mellitus for 10 years. She had no history of HBV or tuberculosis infection.
Physical examination
Physical examination revealed no abnormalities.
Laboratory examinations
Routine laboratory tests revealed normal whole blood cell counts and albumin/globulin ratio 0.9 (normal range, 1.5–2.5). All liver functional tests were normal, including serum aspartate aminotransferase (AST), alanine transaminase (ALT), total bilirubin (TBil) and prothrombin time (PT). An antinuclear antibody-immunofluorescence (ANA-IF) test was positive for nucleolar type at 1:640 dilution and for cytoplasmic type at 1:80 dilution. Tumor marker titers, including α-fetoprotein (AFP), carbohydrate antigen 242 (CA242), carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were within the normal range. Hepatopathic autoimmune antibodies (SLA, AMA, PCA, LKM, HRA, AMA-M2, and ACA) were negative, while SMA was positive at 1:320 dilution, and serum immunoglobulin IgG (18.2 g/L, normal 7–17 g/L) and IgA (4.58 g/L, normal 0.7–3.8 g/L) were slightly elevated. Antibodies against extractable nuclear antigens (ENA) including Smith, RNP, Ro (SSA), La (SSB), Scl-70, Jo-1, rRNP, and antibodies against neutrophil cytoplasmic antibodies (ANCA) including RP3-ANCA, IF-ANCA and MPO-ANCA, were negative. Thyroid parameters were within normal range.
Imaging examinations
The details of this neoplasm are displayed in dynamic CT scan and MRI (Figures 1,2). The nodule was significantly enhanced in the arterial phase, followed by a slight attenuation in the portal phase. MRI suggested differentiation of this small hypervascular lesion from hepatocellular carcinoma (HCC), focal nodular hyperplasia (FNH) and adenoma. Based on radiographic observation indicating malignancy, diagnosis of HCC was considered.
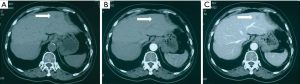
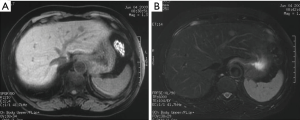
Diagnosis before surgery
The diagnosis of the presented case was HCC before surgery.
Treatment
The patient underwent partial resection of the left lateral hepatic lobe with cholecystectomy.
Outcome and follow-up
The resected liver tissue exhibited a normal smooth surface appearance. A gray-white, well-circumscribed firm nodule 1.2×0.7×1 cm3 in size was located within the removed section. The remaining hepatic parenchyma presented a gray-brown appearance, solid but relatively soft in quality.
The S2 nodule was composed of many lymphoid follicles with large germinal centers containing small polymorphic lymphocytes. Peri-nodular hepatocytes in the peripheral hepatic tissue were partially swollen. Infiltration of a few lymphocytes and local bile ductile hyperplasia were observed in the portal area. A gallstone was found in the gall bladder along with multiple cholesterol polyps, and chronic cholecystitis.
Immunohistochemical studies indicated intrafollicular lymphocytes were Bcl-2-negative, CD10-positive, CD68-positive, CD3-positive and CD20-positive. The germinal center Ki-67 index was 90%. These findings suggested polyclonal origins of the infiltrating lymphocytes.
The patient received routine postoperative anti-infection agents, nutritional support and liver protection treatments. No recurrence or other combination were observed during 6-months of follow-up.
Discussion
RLH is a small, benign, tumorous lesion rarely found in the liver, but more often in other organs, including the gastrointestinal tract, orbit, lung and skin (1). From 1981 to the end of 2015, there have been fewer than 60 cases of hepatic RLH reported in the literature (2,3), suggesting its extreme rarity. Nevertheless, the real incidence of hepatic RLH may be higher than is reported in the literature, due to insignificant symptoms and failure to detect or diagnose. Interestingly, increasing numbers of RLH reports have been published in recent years, suggesting an improvement in the differential diagnosis of hepatic tumor mass and the advancement of laboratory and imaging examination.
RLH usually presents as a single small nodule, ranging from 4–27 mm in size, and mostly occurs in middle-aged women with an average age of 56.8 (range, 15–77) years old in East Asian countries. Hepatic RLH resembles HCC or metastatic neoplasm in liver on imaging findings. Various imaging methods, including US, CT, MRI and PET have been commonly used for diagnosis and differentiation from HCC or metastasis (4-6). RLH lesions often appear as hypoechoic masses on abdominal US (7-9). Plain CT generally reveals areas of low-density compared to the surrounding hepatic tissue. Contrast CT generally reveals tumor enhancement in the arterial phase with substantial attenuation in sequential phases, or no enhancement in any phase. A majority of MRI results reveal a low signal intensity lesion in the T1-weighted image and a high signal intensity lesion in the T2-weighted image (10,11). Hepatic artery angiography reveals a tumor staining in the corresponding area, indicating its hypervascularity (10,11). Some patients are considered for CT arterial portography (CTAP), during which a perfusion defect is often identified (8,12). A tumor-rim was found in 6 cases via various radiographic modalities (10,11). Radiological findings are also comparable with those of vascular hepatic tumors, including HCC or metastasis. Therefore, patients are often misdiagnosed as having HCC or metastatic tumors (10,11,13). There is a lack of efficiency in hepatic RLH differentiation from malignancy using current imaging approaches.
The pathogenesis of RLH remains unclear. An association between the development of hepatic RLH and systemic or local immunological abnormalities has been suggested in earlier reports (14,15). In fact, some cases have been complicated with immunologic abnormalities such as autoimmune thyroiditis, Sjogren’s syndrome, CREST and primary biliary cirrhosis (10-12), while other reported cases have been complicated with cancer, such as gastric cancer, renal cell cancer and colon cancer, suggesting a potential association between RLH and malignant tumors (16,17). Furthermore, 4 cases of hepatic RLH were diagnosed in hepatitis patients (ADIH, CHB, and CHC). Lymphoproliferative diseases have been reported to be associated with chronic liver disease. Lymphocyte infiltration in the portal tracts is frequently observed in chronic liver diseases such as chronic active hepatitis (CAH) and liver cirrhosis, thus supporting the hypothesis of continuity between CAH and pseudolymphoma in RLH development and progression (18).
The unique properties of this case, compared with previous reports, include negative history of chronic hepatitis or other liver disease, positive ANA and SMA test, negative laboratory and histologic findings that did not indicate autoimmune hepatitis, primary biliary cirrhosis or other systemic autoimmune disease. Nevertheless, considering the fact that positive ANA and SMA results are indeed indicating factors for diagnosis of autoimmune hepatitis, it is also possible that the patient was in a very early asymptomatic stage of autoimmune hepatitis development; therefore, long-term follow up is required. However, within 6 months of the surgery, the patient did not exhibit any symptoms relevant to autoimmune hepatitis; further follow-up is underway. Another possibility is that the positive ANA and SMA tests could be specific autoimmune biomarkers for some subtype of RLH in liver, perhaps useful for diagnosis and differentiation of hepatic RLH. Due to the lack of data on ANA and SMA in hepatic RLH in previous studies, more tests on ANA and SMA are needed to further validate their correlation with hepatic RLH.
In respect to treatment, partial hepatectomy is often considered in most cases. If established RLH diagnosis is obtainable before surgery, conservative treatments, including attempted treatment of primary disease (autoimmune abnormality or chronic hepatitis) with close observation of disease progression from RLH to malignancy is plausible.
The differential diagnosis includes inflammatory pseudotumor (IPT) and mucosa-associated lymphoid tissue (MALT) lymphoma. RLH usually lacks typical clinical and pathologic features of IPT, including fever, myofibroblastic proliferation, xanthogranulomatous change and occlusive portal phlebitis (19). Primary MALT lymphoma of the liver is also rare, most characteristic of clonal B-lymphoproliferative disorder. Evidence of B cell clonality is based on immunoglobulin light chain restriction detected through immunohistochemistry. Only in a single case was clonality of lymphocytes assessed through molecular gene rearrangement studies (20). Our immunohistochemical tests indicated germinal centers to be negative for Bcl-2, with mixed infiltrates of B- and T-lymphocytes, indicating that they were reactive and nonneoplastic. Polymerase chain reaction (PCR) analysis for the immunoglobulin gene is useful for detecting clonal B-cell populations. PCR analysis may be considered if immunohistochemistry does not provide enough evidence to prove polyclonality.
Generally, RLH is difficult to diagnose before surgery and without histological testing. Autoimmune antibodies, including ANA and SMA, can be tested before surgery and may provide some useful information regarding diagnosis and treatment. RLH is considered a benign process; patients generally experience smooth uneventful postoperative recovery with no recurrence. Nevertheless, the risk of malignant transformation to lymphoma has been speculated. Therefore, regular follow-up of hepatic RLH patients is crucial for the prevention of potential development of lymphoma.
Conclusions
Hepatic RLH is a rare disease most commonly seen in middle-aged females, characterized by small lesion size, concomitant immunological disorder or carcinoma, and malignant tumor-like radiographic findings. Comprehensive examinations, including autoimmune antibody tests and differential diagnosis should be performed to achieve the diagnosis of RLH. Surgical intervention and histological diagnosis are currently the standard means of accurate treatment and diagnosis.
Acknowledgments
We would like to acknowledge the patient and her family for allowing us to use her medical records in the case report and allowing this case to be submitted and published.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.32). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amer A, Mafeld S, Saeed D, et al. Reactive lymphoid hyperplasia of the liver and pancreas. A report of two cases and a comprehensive review of the literature. Clin Res Hepatol Gastroenterol 2012;36:e71-80. [Crossref] [PubMed]
- Higashi T, Hashimoto D, Hayashi H, et al. Reactive lymphoid hyperplasia of the liver requires differential diagnosis of hepatocellular carcinoma. Surg Case Rep 2015;1:31. [Crossref] [PubMed]
- Sonomura T, Anami S, Takeuchi T, et al. Reactive lymphoid hyperplasia of the liver: Perinodular enhancement on contrast-enhanced computed tomography and magnetic resonance imaging. World J Gastroenterol 2015;21:6759-63. [Crossref] [PubMed]
- Ahmad A, Reha J, Saied A, et al. Association of primary tumor lymph node ratio with burden of liver metastases and survival in stage IV colorectal cancer. Hepatobiliary Surg Nutr 2017;6:154-61. [PubMed]
- Ansari D, Toren W, Andersson R. Primary lymph node ratio and hepatic resection for colorectal metastases. Hepatobiliary Surg Nutr 2018;7:149-50. [Crossref] [PubMed]
- Brudvik KW, Soreide K. Association between the lymph node ratio and hepatic tumor burden: importance for resectable colorectal liver metastases? Hepatobiliary Surg Nutr 2018;7:206-8. [Crossref] [PubMed]
- Jimenez R, Beguiristain A, Ruiz-Montesinos I, et al. Image of the month. Reactive lymphoid hyperplasia. Arch Surg 2008;143:805-6. [PubMed]
- Maehara N, Chijiiwa K, Makino I, et al. Segmentectomy for reactive lymphoid hyperplasia of the liver: Report of a case. Surg Today 2006;36:1019-23. [Crossref] [PubMed]
- Matsumoto N, Ogawa M, Kawabata M, et al. Pseudolymphoma of the liver: Sonographic findings and review of the literature. J Clin Ultrasound 2007;35:284-8. [Crossref] [PubMed]
- Kobayashi A, Oda T, Fukunaga K, et al. MR imaging of reactive lymphoid hyperplasia of the liver. J Gastrointest Surg 2011;15:1282-5. [Crossref] [PubMed]
- Yoshikawa K, Konisi M, Kinoshita T, et al. Reactive lymphoid hyperplasia of the liver: literature review and 3 case reports. Hepatogastroenterology 2011;58:1349-53. [Crossref] [PubMed]
- Machida T, Takahashi T, Itoh T, et al. Reactive lymphoid hyperplasia of the liver: a case report and review of literature. World J Gastroenterol 2007;13:5403-7. [Crossref] [PubMed]
- Fukuo Y, Shibuya T, Fukumura Y, et al. Reactive lymphoid hyperplasia of the liver associated with primary biliary cirrhosis. Med Sci Monit 2010;16:CS81-6. [PubMed]
- Kwon YK, Jha RC, Etesami K, et al. Pseudolymphoma (reactive lymphoid hyperplasia) of the liver: A clinical challenge. World J Hepatol 2015;7:2696-702. [Crossref] [PubMed]
- Nagano K, Fukuda Y, Nakano I, et al. CASE REPORT: Reactive lymphoid hyperplasia of liver coexisting with chronic thyroiditis: Radiographical characteristics of the disorder. J Gastroenterol Hepatol 1999;14:163-7. [Crossref] [PubMed]
- Park HS, Jang KY, Kim YK, et al. Histiocyte-rich reactive lymphoid hyperplasia of the liver: unusual morphologic features. J Korean Med Sci 2008;23:156-60. [Crossref] [PubMed]
- Takahashi H, Sawai H, Matsuo Y, et al. Reactive lymphoid hyperplasia of the liver in a patient with colon cancer: report of two cases. BMC Gastroenterol 2006;6:25. [Crossref] [PubMed]
- Kim SR, Hayashi Y, Kang KB, et al. A case of pseudolymphoma of the liver with chronic hepatitis C. J Hepatol 1997;26:209-14. [Crossref] [PubMed]
- Sato K, Ueda Y, Yokoi M, et al. Reactive lymphoid hyperplasia of the liver in a patient with multiple carcinomas: a case report and brief review. J Clin Pathol 2006;59:990-2. [Crossref] [PubMed]
- Willenbrock K, Kriener S, Oeschger S, et al. Nodular lymphoid lesion of the liver with simultaneous focal nodular hyperplasia and hemangioma: discrimination from primary hepatic MALT-type non-Hodgkin's lymphoma. Virchows Arch 2006;448:223-7. [Crossref] [PubMed]


