
Effects of okadaic acid and hematein on human lung adenocarcinoma A549 cells and responses of mitochondria and endoplasmic reticulum apoptosis pathways
Introduction
At the current stage, more than 1.8 million new lung cancer cases were diagnosed each year (1), and more than one million die of lung cancer each year worldwide. Chemotherapy, radiotherapy and surgery are generally the primary treatments, and can effectively block proliferation of tumor cells, extending patient’s life indefinitely. However, the side effects, such as lusterless complexion, shortness of breath, fatigue, loss of appetite, low white blood cells, easy infection, baldness, severely lower the quality of patient’s life. To reduce the unwanted side effects, increasing attentions have been focused on new anti-cancer medicines.
Cisplatin is currently the most effective drug for non-small cell lung cancer (NSCLC), yet the efficiency of platinum-based chemotherapy is only 30% to 60% (2). Moreover, the harmful side effects make patients suffer from nausea, vomiting, nephrotoxicity, neurotoxicity, and myelosuppression (2). Recently, more and more biological drugs, such as paclitaxel, docetaxel, okadaic acid (OA) and curcumin were used to decrease the side effects of cisplatin by drug combination (3-5). These drugs are closely related to traditional Chinese medicine (TCM), which has been attracting attentions worldwide because of its anticancer effects and low side effects. TCM is a kind of natural medicines, including herbs, animals and mineral. Although TCM hard to effectively inhibit or kill tumour cells as western medicines, a large number of reports suggested that TCM combined other anti-cancer medicines is an efficient method to treat cancer (6,7). To date, grosvenorii, quinquefolium, and tripterygium have been found of strong anti-cancer effects (8-10).
Hematein is an oxidation product of hematoxylin, which can be extracted from haematoxylon campechianum, and has usually been used as an anti-tumor drug, antibacterial drug, and anti-inflammatory drug (11-13). Hematein has been demonstrated able to inhibit proliferation of cancer cells and induce cell apoptosis, such as breast cancer MCF-7 cells and A549 cells (14,15), and has a low toxicity to normal cells, showing a high anti-cancer potential. OA is mainly produced by Prorocentrum lima, is a long-chain fatty acid, and belongs to a diarrhea shellfish toxin. It is a strong carcinogen and poses a serious threat to human health through diet (16). However, increasing research found that OA or combined with other anti-cancer drugs can effectively inhibit rat mesangial cells, human amniotic cells, liver cancer cells, lung cancer cells. Although OA and hematein could inhibit proliferation and induce apoptosis of A549 cells, respectively, their combined effects on proliferation and apoptosis of A549 cells are yet unknown. In this study, MTT assay was employed to investigate the combined effects of OA and hematein on proliferation of A549 cells. Inverted phase contrast microscope, scanning electron microscope (SEM), and western blot were used to reveal the combined effects of OA and hematein on apoptosis of A549 cells. The results benefit exploring new and more effective anti-cancer drugs.
Methods
A549 cells and reagents
A549 cells was purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Beijing, China). OA (C44H68O13) was purchased from Sigma (St. Louis, MO, USA). Hematein (C16H12O6) was purchased from Santacruze (Shanghai, China). RPMI-1640 medium was purchased from Gibco (Grand Island, NY, USA). MTT and DMSO were purchased from Solarbio (Beijing, China). The ECL detection kit was purchased from Pharmacia Biotech (Uppsala, Sweden). The antibody of Bcl-2, Bax and Cytochrome C was purchased from Proteintech (Chicago, USA). The antibody of Chop, Calpain2, JNK1 and IRE1 was purchased from Boaosen Biotechnology Co., Ltd (Beijing, China).
Cell culture
The A549 cells were cultured using RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 100 IU/mL penicillin, 100 µg/mL streptomycin and 10% heat-inactivated newborn fetal calf serum in a CO2 incubator at 37 °C (Sanyo Electronics Co. Ltd., Japan).
Determinations of cell cytotoxicity
Cell cytotoxicity of OA, Hematein and their combination was evaluated by an MTT (Beijing Solarbio Technology Co., Ltd.) assay according to the method described previously (17). The experimental group was divided into blank group, control group, solvent control group and 9 concentration gradient experimental groups. Each group was set with 8 replicates. The cell suspension in logarithmic phase was diluted to 4,000 cells/mL, and then was seeded into a 96-well plate with 100 µL per well. After 24 h cultivation, OA (10–90 ng/mL), hematein (5–100 µg/mL) and hematein (20 µg/mL) combined with OA (10, 20, 40 ng/mL) were added into the wells respectively. After cultivation for 4 h, 20 µL MTT (5 mg/mL) was added into each well, followed by incubation for 4 h. The medium was removed, 150 µL DMSO (Beijing Solarbio Technology Co., Ltd.) was added into each well. The optical density (OD) value was detected at 570 nm in a microplate reader (BIO-RAD, Japan). The group without solvent and drugs was set as blank (B), and group with solvent and drugs was set as solvent control (C) and drug treatment (A) respectively. The entire process was performance in darkness. Cell viability = (A–B)/(C–B) ×100%.
Morphological observation of A549 cells
Inverted phase contrast microscope, fluorescent inverted microscope and SEM were used to detect the morphological changes of A549 cells with or without drugs treatment. For the inverted phase contrast microscope analysis, the cells were treated with the method mentioned above. For fluorescent inverted microscope analysis, acridine orange that can bind to double DNA was added into the treated and untreated A549 cells (104 cells/mL) inoculated in a 6-well plate, and then was set in darkness at room temperature for 0.5 h. Subsequently, the cells were washed 3 times with PBS buffer, and observed under fluorescent inverted microscope (Cairns, Germany). For SEM analysis, the drug treated and untreated cells were harvested and washed 3 times with 0.1M PBS buffer, then, fixed in glutaraldehyde solution at 4 °C for 3 h. After fixation, the samples were washed with PBS, and dehydrated using with different concentrations of ethanol, followed by displacing with isoamyl acetate for 30 min and drying in a dryness oven for 3–4 h. Finally, the cells were visualized under a JSM-6700F SEM (JEOL, Japan).
Detection of mitochondrial depolarization and reactive oxygen species (ROS) levels
JC-1 Mitochondrial Membrane Potential Assay Kit was used to detect mitochondrial depolarization of the drug treated and untreated A549 cells (10 ng/mL OA, 20 µg/mL hematein, and their combination) according to the kit instruction. F-380 fluorescence spectrophotometer (Tianjin Gangdong Technology Development Co., Ltd., China) was used to detect the OD value. The intracellular ROS level was measured by non-fluorescent DCFH-DA (2',7'-dichloro-fluorescein diacetate) oxidation to fluorescent DCF using a ROS assay kit (Beyotime S0033). The fluorescence values were measured in F-380 fluorescence spectrophotometer with excitation wavelength of 488 nm and emission wavelength of 525 nm.
SDS-PAGE and Western blot
Total cell proteins were extracted by conventional methods. The collected proteins (40 µg) was fully denatured by heating at 95–100 °C for 10 min, then were loaded on SDS-PAGE. The proteins were separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA). They were incubated in a blocking solution consisting of 5% skim milk powder in 0.1% Tween-20 PBS (PBS-Tween) overnight with specific antibodies (diluted 1:500 to 10,000). The membrane was then washed at least 3 times and then incubated in PBS-Tween supplemented with a second antibody (containing horseradish peroxidase, diluted 1:5,000) for 2 h. Finally, the membrane was washed again and the protein recognized by the antibody was visualized using the ECL detection kit (Pharmacia Biotech). The drop was placed on the front of the nitrocellulose (NC) filter membrane for about 1 min, placed in an X-ray film holder, and developed and fixed in a dark room.
Statistical analysis
ANOVA based on Duncan’s multiple range test was performed to reveal the differences between the control and drugs treated groups in SPSS 17.0.
Results
Proliferation of A549 cells under drugs treatment
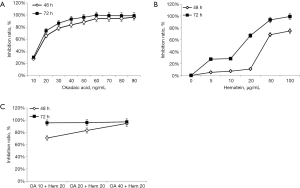
MTT assay showed that both OA and hematein effectively inhibited the proliferation of A549 cells, showing a time-dose dependent manner (Figure 1). When OA exceeded 30 ng/mL, A549 cells were significantly inhibited, with the inhibition ratio of 78.3% and 86.3% at 48 h and 72 h, respectively, while 50 µg/mL hematein significantly inhibited A549 cells, with the inhibition ratio of 68.5% and 93.4% at 48 h and 72 h, respectively. Compared with single drug treatment, the combined drugs with lower dosage (OA 10 ng/mL and hematein 20 µg/mL) also achieved the same inhibitory effect, with the inhibition ratio up to 73.6% and 94.5% at 48 h and 72 h, respectively. According to Kim’s formula, it suggested that OA and hematein had synergistic inhibitory effects on A549 cell proliferation. Compared to the single drug treated groups (OA 20 ng/mL, hematein 50 µg/mL), 10 ng/mL OA and 20 µg/mL hematein treatment could reach a similar inhibitory ratio.
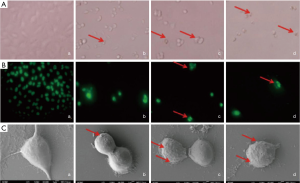
Cell morphology
Figure 2A showed that the cell morphology of drug treated A549 cells was significantly changed compared to the control group. The cells were spindle-shaped, and contacted with each other in the control group, whereas, the cells became round or oval (indicated by the arrows), and detached from each other in the drug treated groups. Moreover, more apoptotic bodies (indicated by the arrows) were observed in OA and hematein treated cells than those in the single drug treated groups, indicating cell apoptosis was more obvious. As shown in Figure 2B, the condensed and shattered chromatin (indicated by the arrows) was significantly observed in drug treated groups. SEM analysis indicated (Figure 2C) the cell bodies became round accompanied by increased and irregular cell surface protrusions (indicated by the arrows) in the drug treated groups.
Mitochondrial depolarization and ROS levels
Apoptosis is frequently associated with depolarization of mitochondrial membrane potential. As Figure 3A showed, JC-1 fluorescence obviously decreased in the OA and hematein treated groups: its red-green fluorescence ratio decreased 0.9 and 0.68 respectively, compared with the control groups. To the groups simultaneously treated by OA and hematein, JC-1 fluorescence decreased 1.73. Compared with the control, the increased OP3 density indicated an increase of ROS levels in the drugs treated group (Figure 3B). Moreover, the ROS level of the combined drugs treated group was significantly higher than that of the control group (P<0.01).

The responses of mitochondrial and endoplasmic reticulum (ER) apoptosis-related proteins
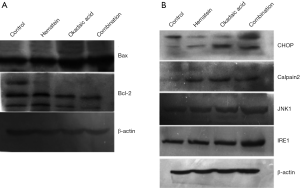
Western blot showed that pro-apoptotic Bax protein that relates to mitochondrial apoptotic pathway obviously increased in the drugs treated groups, particularly, the groups treated by drugs combination, and the anti-apoptotic protein Bcl-2 decreased (Figure 4A). The results indicated that mitochondrial apoptotic pathway involved in A549 cell apoptosis induced by the drugs. At the same time, the ER apoptosis-related proteins CHOP, Calpain2, JNK1, and IRE1 significantly increased in the drug treated groups, and the groups treated by drugs combination had the highest protein expression (Figure 4B), indicating that ER apoptotic pathway also played an important role in the drugs induced apoptosis of A549 cells.
Discussion
Lung cancer has high morbidity and mortality worldwide (18). Although chemotherapy, in particular cisplatin, is one of the most effective treatment for lung cancer (19,20), the side effects on patient’s life quality cannot be ignored. Therefore, increasing efforts have been devoted to the development of new anti-cancer drugs with low toxicity on normal cells (21). Recently, natural anti-cancer medicines have attracted more and more attentions. Some natural medicines, such as grosvenorii, quinquefolium, and garlic, OA, and hematein, were found of strong anti-cancer effects (9,10,22).
This study focused on the combined effects of natural medicines OA and hematein on proliferation and apoptosis of A549 cells. OA is a marine toxin separated from Prorocentrum lima (23), and is of anti-tumor effect at a low dose (24). Recent researches had demonstrated that OA can induce apoptosis of many cell types, including A549 cells, neurotoxicities, human intestinal cell lines, human retinoblastoma Y79 cells (17,25-27). Moreover, it can reduce the side effects of cisplatin, and improve the therapeutic effect on tumors when was combined with cisplatin (28,29). Hematein is an oxidation product of hematoxylin, and was demonstrated able to inhibit cancer cells (11,30). As far as we know, there has no report on the combined effects of OA and hematein on A549 cells.
The results here showed that 30 ng/mL OA and 50 µg/mL hematein could significantly inhibit the proliferation of A549 cells. The proliferation ration is very close to the treatment effect of platinum-based chemotherapy. Importantly, lower dosage (10 ng/mL OA and 20 µg/mL hematein) could achieve the same inhibitory effect when were used in combination. According to Kim's formula, OA and hematein had synergistic inhibitory effects on A549 cell proliferation.
Inducing cell apoptosis is another main pathway of anti-cancer drugs to inhibit proliferation of cancer cells (31). Therefore, cell apoptosis of A549 cells under OA and hematein treatment was investigated. Using inverted phase contrast microscope and SEM, apoptotic bodies and damaged cells were obviously observed under the drugs treated groups, in particular the groups with combined drugs treatment. Mitochondrial apoptosis pathway and ER apoptosis pathway are the key pathways in inducing cell apoptosis. As expected, the intracellular ROS level was significantly elevated and mitochondrial membrane potential decreased in the drugs treated groups. Western blot showed that pro-apoptotic protein Bax increased, while the anti-apoptotic protein Bcl-2 decreased. In addition, the ER apoptosis-related proteins CHOP, Calpain2, JNK1 and IRE1 significantly increased in the drugs treated groups. In addition, it suggested that the drugs combination could aggravate the apoptosis of A549 cells compared with the single drug treated group.
Conclusions
In summary, this study investigated the combined effects of OA and hematein on A549 cells from perspectives of cell proliferation and cell apoptosis. The results demonstrated the higher anti-cancer effects of the drugs combination than the single drug, and revealed that mitochondria and ER apoptosis pathways involved in apoptosis of A549 cells.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of College of Life Sciences, Qufu Normal University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351-71. [Crossref] [PubMed]
- Effects of paclitaxel on cell proliferation and apoptosis and its mechanism in human lung adenocarcinoma A549 cells. J Nanjing Medical Univ 2006;20:360-4.
- Elattar TM, Virji AS. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res 2000;20:1733-8. [PubMed]
- Liu X, Chang Q, Zhao X, et al. Establishment of Docetaxel Resistant Variant of Human Lung Adenocarcinoma Cell Line A549/DTX and Its Resistance Mechanism. Cancer Res Prev Treat 2014;41:519-22.
- Bian L, Tian SS, Chen YL. Progress in the research on commonly used anti-cancer traditional Chinese medicine capsules combined with chemotherapy on middle-advanced stage lung cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009;29:279-82. [PubMed]
- Hu W, Ding JY, Jia SX, et al. ShenMai Injection Combined with Chemotherapy in the Treatment of Advanced Lung Cancer. J Liaoning Univ Tradit Chin Med 2008;108-9.
- Yang HS, Kim JY, Lee JH, et al. Celastrol isolated from Tripterygium regelii induces apoptosis through both caspase-dependent and -independent pathways in human breast cancer cells. Food Chem Toxicol 2011;49:527-32. [Crossref] [PubMed]
- Liu C, Cai TY, Zhao XM, et al. Apoptosis effect of Siraitia grosvenorii extracts on lung cancer cells A549 and its mechanisms. Chin Pharmacol Bull 2015;31:1310-4. [Crossref]
- Yin HJ, Zhang Y, Jiang YR. Effect of folium panax quinquefolium saponins on apoptosis of cardiac muscle cells and apoptosis-related gene expression in rats with acute myocardial infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi 2005;25:232-5. [PubMed]
- Kim KJ, Yoon KY, Yoon HS, et al. Brazilein Suppresses Inflammation through Inactivation of IRAK4-NF-kappaB Pathway in LPS-Induced Raw264.7 Macrophage Cells. Int J Mol Sci 2015;16:27589-98. [Crossref] [PubMed]
- Choi JH, Jeong TS, Kim DY, et al. Hematein inhibits atherosclerosis by inhibition of reactive oxygen generation and NF-kappaB-dependent inflammatory mediators in hyperlipidemic mice. J Cardiovasc Pharmacol 2003;42:287-95. [Crossref] [PubMed]
- Hung MS, Xu Z, Chen Y, et al. Hematein, a casein kinase II inhibitor, inhibits lung cancer tumor growth in a murine xenograft model. Int J Oncol 2013;43:1517-22. [Crossref] [PubMed]
- Tao L, Li J, Zhang J. Brazilein induced cells apoptosis in human lung cancer A549 clls and its effects on endoplasmic reticulum stress. Chongqing Med 2015;44:4180-2.
- Tao LY, Li JY, Zhang JY. Brazilein Induced Cells Apoptosis in Human Breast Cancer MCF-7 Cells and Its Action Mechanism. J Sun Yat-Sen Univ 2011;32:449-53. (Med Sci).
- Lu SY, Liu ZS, Zhou Y, et al. ELISA and HPLC-MS/MS for Detecting Okadaic Acid in Shellfish. Food Sci 2009;30:158-62.
- Wang R, Lv L, Zhao Y, et al. Okadaic acid inhibits cell multiplication and induces apoptosis in a549 cells, a human lung adenocarcinoma cell line. Int J Clin Exp Med 2014;7:2025-30. [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol 1990;40:2353-62. [Crossref] [PubMed]
- Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 2000;275:39435-43. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Zhang X, Zhu Y, Duan W, et al. Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol Med Rep 2015;11:2755-60. [Crossref] [PubMed]
- Song Q, Baxter GD, Kovacs EM, et al. Inhibition of apoptosis in human tumour cells by okadaic acid. J Cell Physiol 1992;153:550-6. [Crossref] [PubMed]
- Lin C, Liu ZS, Tan CY, et al. Contamination of commercially available seafood by key diarrhetic shellfish poisons along the coast of China. Environ Sci Pollut Res Int 2015;22:1545-53. [Crossref] [PubMed]
- Di Fiore R, Drago-Ferrante R, D'Anneo A, et al. In human retinoblastoma Y79 cells okadaic acid-parthenolide co-treatment induces synergistic apoptotic effects, with PTEN as a key player. Cancer Biol Ther 2013;14:922-31. [Crossref] [PubMed]
- Ferron PJ, Hogeveen K, Fessard V, et al. Comparative analysis of the cytotoxic effects of okadaic acid-group toxins on human intestinal cell lines. Mar Drugs 2014;12:4616-34. [Crossref] [PubMed]
- Zhao J, Wang D, Li L, et al. Protective effects of humanin on okadaic Acid-induced neurotoxicities in cultured cortical neurons. Neurochem Res 2014;39:2150-9. [Crossref] [PubMed]
- Youn MJ, So HS, Cho HJ, et al. Berberine, a natural product, combined with cisplatin enhanced apoptosis through a mitochondria/caspase-mediated pathway in HeLa cells. Biol Pharm Bull 2008;31:789-95. [Crossref] [PubMed]
- Nakaoka T, Ota A, Ono T, et al. Combined arsenic trioxide-cisplatin treatment enhances apoptosis in oral squamous cell carcinoma cells. Cell Oncol (Dordr) 2014;37:119-29. [Crossref] [PubMed]
- Zhong X, Wu B, Pan YJ, et al. Brazilein inhibits survivin protein and mRNA expression and induces apoptosis in hepatocellular carcinoma HepG2 cells. Neoplasma 2009;56:387-92. [Crossref] [PubMed]
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal anti-inflammatory drugs (NSAIDs). Br J Dermatol 2007;156:25-33. [Crossref] [PubMed]


