
Association of epidermal growth factor receptor protein expression with histopathological and clinical parameters in carcinoma of the larynx
Introduction
Laryngeal carcinoma is the third most common head and neck malignancy after oral cavity and pharynx cancer (1). Approximately 85–95% of laryngeal tumors are squamous cell carcinomas and 60% of patients present in the advanced stages (2) at the time of diagnosis. Laryngeal tumors are more prevalent in men than women (3) and ethnic variations have been observed in different populations with African Americans showing a higher incidence compared to Caucasians (4).
Alcohol consumption and tobacco usage is the major etiology accounting for 80% of head and neck squamous cell carcinomas (HNSCC) (5). Consequently, the global incidence of HNSCC is 4.6% and among HNSCC the oral cavity and larynx cancer incidence is 42.5% and 21.2%, respectively (6). Human papillomavirus (HPV) infection is also a risk factor in HNSCC, especially as it is associated with oropharyngeal cancer compared to other subsites of HNSCC (7).
The overall goal of therapeutic management of laryngeal carcinoma is to preserve the function of the larynx and in order to attain this it is important to assess the recurrence risk and prognosis of the disease in each case (8). Chemo-resistance is a major obstacle in the management of the disease (9). Even with the availability of various treatment modalities for laryngeal carcinoma, the five-year survival rate has declined in the past four decades (2). Tumor management by chemotherapy and radiotherapy has become feasible options and for localized tumors, surgery is the standard treatment plan. In the event of metastasis and for organ preservation, novel therapeutic agents will play a potential role in addressing laryngeal carcinoma (2).
The genetic alterations in the form of mutations and varied expression patterns in the oncogenes and tumor suppressor genes have gained importance in cancer management and for the designing of targeted therapy. These genetic alterations in laryngeal and oral cancer are complex and interrelated. Activation of proto-oncogenes, inactivation of tumor suppressor genes and altered expression of both genes play a major role in the prognosis and recurrence rate (10).
The epidermal growth factor receptor (EGFR), a cell surface receptor, was for the first time reported to be associated with HNSCC in 1986 (11). Its activation and over expression transform the downstream functions, such as cell proliferation, metastasis and angiogenesis. EGF and transforming growth factorβ promote epithelial to mesenchymal transition (EMT) which is a dynamic cellular process essential for the metastasis development by regulating a specific set of transcription factors (12). The abnormal expression of EGFR protein has been observed in several cancers and has gained importance in terms of drug design to treat various cancers by anti-EGFR molecules. Monoclonal antibodies (Cetuximab, Panitumumab) and small molecule tyrosine kinase inhibitors (Gefitinib, Erlotinib, Lapatinib, Canertinib) are used in the treatment of various cancers including colorectal cancer and HNSCC for the former and non-small cell lung cancer and pancreatic cancer for the latter (13).
Phosphatase and tensin homolog (PTEN) and p16INKA are tumor suppressor genes. PTEN regulates phosphoinositide 3-kinase signaling pathway by dephosphorylating the lipid signaling intermediate phosphatidylinositol-3,4,5-triphosphate (PIP3). Due to the altered expression or loss of PTEN gene function, elevated PIP3 is observed in cells. The increase in PIP3 concentration leads to cell survival, growth and proliferation (14). PTEN loss of function is observed as a result of transcriptional silencing, somatic mutations and deletions (15). PTEN reduced expression also plays a role in cancer susceptibility (16). P16INKA is a member of Ink4 family of cyclin dependent kinase inhibitors. P16INKA acts as a negative regulator of the cell cycle by “S phase” inhibition (17). Genetic alterations and the over expression of p16INKA play an essential role in several tumors, such as HNSCC, breast, colon, skin, bladder and cervix (18). The development of biomarker signatures may pave the way for novel treatment regimens to treat laryngeal carcinoma. Hence, this study is focused on analyzing the expression of EGFR, p16 INKA, PTEN and HPV infection to determine the association of these markers with histopathological parameters and prognosis in laryngeal carcinoma patients.
Methods
Study cohort
A total of 18 formalin fixed paraffin embedded (FFPE) tissue samples from Saudi patients with confirmed laryngeal carcinoma were obtained from King Fahd Hospital of the University. The FFPE blocks were reanalyzed by hematoxylin and eosin (HE) staining to reconfirm the diagnosis and to check for tumor content. This study was approved by the Institutional Review Board of Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia (IRB number: IRB-2014-08-044).
Immunohistochemistry
FFPE sections of 5 µm thickness were cut using Leica microtome (Leica, USA) and these sections were mounted on poly-L-lysine coated slides (Sigma, USA). The slides were incubated at 60 °C for 12 hours, deparaffinization was carried out using xylene and rehydrated with various concentrations of ethanol. Primary blocking was performed by incubating in 3% hydrogen peroxide (Sigma, USA) solution for 30 minutes. Antigen retrieval was performed by a heat inducing method using a microwave. Secondary blocking reaction was carried out by incubating in 3% bovine serum albumin solution (Sigma, USA) for 30 minutes. Primary monoclonal antibodies, PTEN (Biocare medical, USA), p16INK4 (Biogenex, USA) and EGFR (Biogenex, USA) were used in different concentrations in the respective reactions as per the manufacturer’s instructions. The slides were incubated with the secondary antibody conjugated with horse radish peroxidase polymer followed by 3,3'-diaminobenzidine (DAB) substrates (Biogenex, USA). Later, the slides were counter stained with hematoxylin, followed by dehydration and the cover slip was mounted on a slide.
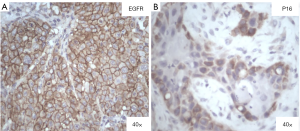
The stained slides were observed under a microscope and each slide was scored based on the membrane staining. The EGFR scoring pattern that was followed is: 0= no staining or weak staining; 1+ = faint staining; 2+ = moderate and 3+ = strong staining of tumor cells. The cases that presented with >10% of staining were considered as having a positive staining (Figure 1). For p16INK4, the nuclear and cytoplasmic staining was considered, and the score was calculated as follows: 0= no staining, 1= weak, 2= intermediate and 3= strong staining of tumor cells. The results were dichotomized as negative (0 and 1) and positive (2 and 3) for p16INK4expression. PTEN expression interpretation was dichotomous (negative and positive) and if a case showed strong positive staining in the majority or all of the tumor cells it was considered to be positive and if tumor cells showed no staining but the adjacent normal cells showed positive staining, it was considered to be negative for PTEN.
In-situ hybridization
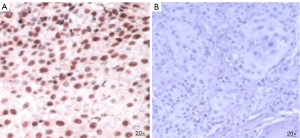
HPV infection in the FFPE tissue sections were detected using in-situ hybridization. Tissue sections were treated with the nucleic acid retrieval system (Biogenex, USA) as per the manufacturer’s instructions to expose the DNA sequence. The slides were then treated with fluorescein labelled probes for HPV16 and 18 (Biogenex, USA) followed by the detection of probe target hybrid by the immunohistochemistry procedure. Anti-fluorescence antibody was added to the slide followed by secondary antibody enzyme conjugate and substrate (DAB). Brown coloration was developed at the location of the probe target hybrid (Figure 2). Interpretation, which was performed by two independent pathologists, resulted in 100 nuclei being counted on each slide.
Data analysis
SPSS version 20 software (IBM, USA) was used to analyze the data, which was represented in category variables and checked for distribution of proportions across category variables using Fisher exact test. The bivariate relationship between the variables, such as smoking status, stage, prognosis, node positivity, HPV status, EGFR, p16INK4 and PTEN protein expression, was determined by Spearman’s rank correlation test. The results were considered significant if the P value was <0.05.
Results
All the samples were obtained from patients who had been clinically diagnosed with carcinoma of the larynx. The mean age of the patient population was 54±10 years. Prognosis was calculated based on the follow-up of the patient for 5 years. Recurrence or death due to disease was considered as a poor prognosis and based on this, 66.7% of the patients had a bad prognosis (Table 1).
Table 1
| Parameter | Classification | Samples, n (%) |
|---|---|---|
| Smoking status | Smokers | 9 (50.0) |
| Non-smokers | 9 (50.0) | |
| Stage | 3 | 8 (44.4) |
| 4 | 10 (55.5) | |
| Node status | Positive | 14 (77.8) |
| Negative | 4 (22.2) | |
| Prognosis | Good | 6 (33.3) |
| Bad | 12 (66.7) | |
| Treatment regimen | RT+CT+S | 7 (38.9) |
| RT+S+CT | 1 (5.6) | |
| S+CT+RT | 4 (22.2) | |
| S+RT+CT | 6 (33.3) | |
| HPV | Positive | 5 (27.8) |
| Negative | 13 (72.2) | |
| EGFR | Positive | 15 (83.3) |
| Negative | 3 (16.7) | |
| P16INK4 | Positive | 10 (55.6) |
| Negative | 8 (44.4) | |
| PTEN | Positive | 1 (5.6) |
| Negative | 17 (94.4) |
RT, radiotherapy; CT, chemotherapy; S, surgery; HPV, human papillomavirus; EGFR, epidermal growth factor receptor; PTEN, phosphatase and tensin homolog.
According to the treatment modality, patients were classified into four groups; (I) 38.9% were treated by radiotherapy and chemotherapy prior to surgery; (II) 5.6% underwent surgery followed by radiotherapy and chemotherapy; (III) 22.2% underwent surgery followed by chemotherapy; and (IV) 33.3% had radiotherapy and surgery followed by chemotherapy and more radiotherapy. HPV and overexpression of EGFR was seen in 27.8% and 83.3% of patients, respectively. Tumor suppressors p16INK4 and PTEN was negatively expressed in 44.4% and 94.4% of patients, respectively. All the HPV positive cases showed over expression of EGFR and negative expression of PTEN. Of the HPV positive cases, 40% presented with p16INK4 negative expression. Lymph node positivity was observed in 80% of the HPV positive cases.
Bivariate analysis revealed that EGFR over expression was associated with lymph node positivity (P=0.045) and stage of the disease (P=0.035) (Table 2). However, the EGFR over expression was not associated with prognosis and smoking status. P16INK4, PTEN protein expression and HPV infection did not yield any significant correlations with smoking status, node positivity, disease stage, treatment regimen and disease prognosis. Disease prognosis was observed to be associated with smoking status (P=0.048).
Table 2
| Clinicopathologic parameters | HPV status | EGFR expression | P16INK4 expression | PTEN expression |
|---|---|---|---|---|
| Smoking status | 0.624 | 0.555 | 1.000 | 0.332 |
| Node positivity | 0.896 | 0.045 | 0.814 | 0.608 |
| Prognosis | 0.486 | 0.201 | 0.201 | 0.496 |
| Stage of the disease | 0.827 | 0.035 | 0.621 | 0.276 |
| Treatment regimen | 0.221 | 0.858 | 0.893 | 0.275 |
HPV, human papillomavirus; EGFR, epidermal growth factor receptor; PTEN, phosphatase and tensin homolog.
Discussion
Majority (95%) of HNSCC cases are squamous cell carcinomas, which advocate HNSCC to be a homogenous disease. However, the various subclasses of HNSCCs differed at histological level and genomic and transcriptome studies revealed molecular heterogeneity in HNSCC (19). TP53 mutations were seen in 39–53% of HNSCC tumors overall and 56.7% in laryngeal carcinoma, these mutations were associated with poor survival (20). NOTCH1 gene inactivating mutations seen in 15% of HNSCC cases and this pathway regulates cell cycle and survival by acting as a tumor suppressor. NOTCH3 gene was associated with poor disease and overall survival in laryngeal carcinoma (21).
DNA damage repair biomarkers including MAP17 and pH2AX overexpression found to be associated with better overall survival and laryngoesophageal dysfunction free survival (22). Nucleotide excision repair (NER) pathway involved in removing DNA cross links caused by chemo and radiotherapy, excision repair cross-complementation group 1 gene mutations of this pathway increase the laryngeal cancer risk (23). Signal transducer and activator of transcription 3 pathway activation regulates cell proliferation and tumor survival and found to be associated in laryngeal cancer (24).
Several studies have emphasized the need for molecular biomarkers to aid in determining the prognosis for patients with HNSCC. However, HNSCC subsite specific data is scarce. Hence, this study focused on carcinoma of the larynx and its association with specific molecular markers, namely EGFR, PTEN expression and HPV infection and p16INK4.
EGFR activation leads to cell proliferation, metastasis, invasion and angiogenesis via tyrosine kinase mediated activation of PI3K, PTEN/AKT, MAPK, ERK and JAK/STAT pathways. Hence, the activation of EGFR is crucial to the carcinogenesis (25,26). EGFR over expression was observed in >80% of HNSCC subjects (13); 68.6% (27) in laryngeal carcinoma and 91.5% in glottic laryngeal carcinoma patients (28). Similarly, our cohort exhibited EGFR over expression in 83.3% of cases. Other studies have attributed EGFR over expression to dephosphorylation and reduced degradation of EGFR protein and/or the high percentage of exon 19 and exon 20 insertions (26,29).
An advanced stage of the disease was associated with EGFR overexpression (P=0.035) in the present study which is consistent with studies conducted by Kontić et al. (27) and Ding (30). EGFR protein expression is elevated in lymph node positive group (P=0.045), which is similar to an earlier study conducted by Ding (30). Clinical correlation of EGFR over expression is the key to finding the association with histopathological parameters, prognosis and survival. Also, this correlation between EGFR over expression at the time of the diagnosis with advanced stage, lymph node involvement aids in the estimation of the tumor progression, metastasis and the potential for recurrence of the disease. Hence, EGFR protein status can be considered as an independent prognostic factor in laryngeal carcinoma.
Loss of PTEN expression is a commonly occurring event in HNSCC, overall, along with PIK3CA mutations (31). The correlation between the PIK3CA mutation and PTEN gene expression was assessed in our previous study (25) in which we reported that 29% of cases presented with PIK3CA mutations and 60% of cases exhibited PTEN gene down regulation in the HNSCC cohort. The present study focused on laryngeal carcinoma and it was found that 94% of cases included in the study presented with low expression of PTEN. However, this percentage is higher than the study by Mastronikolis et al. (32) which reported that 56% of laryngeal tumors had abnormal (low) PTEN expression. In our study, the high percentage of low expression of PTEN may be due to the advanced grade of the tumors. However, we did not find any significant correlation with the prognosis of the disease and node positivity.
HPV is known to be involved in the development of cervical cancer (33). The mechanism of carcinogenesis begins with the viral oncogenes E6 and E7 which inactivate the retinoblastoma and tumor protein 53, which in turn causes cell cycle dysregulations and leads to carcinogenesis (34). Further research has revealed HPV involvement in HNSCC and the presence of HPV increases the risk of HNSCC (35). In the subtypes of HNSCC, HPV infection was seen less frequently in laryngeal cancers and oral cavity cancers compared to oropharyngeal cancers (7,36). In Laryngeal carcinoma, the average prevalence of HPV is 28% (ranging from 6.8% to 58.8%) (37-40). The wide range of HPV prevalence is due to the non-specificity of detection method used in these studies. The availability of novel sensitive technologies (in-situ hybridization) to detect HPV is also the reason for the reduced prevalence of HPV.
The combination of testing of HPV and p16INK4 is now promoted extensively as marker for disease progression (41). In the present study, this algorithm was employed by using highly sensitive technique in-situ hybridization to detect the HPV infection in tumor samples along with p16INK4 expression profiling. In the present study, we observed that 27.8% of laryngeal cancer cases presented with HPV infection. Among the HPV positive cases, 60% showed high p16INK4 protein expression. Based on our results, we postulated that there is a weak correlation between the HPV infection with p16INK4 with the prognosis unlike in oropharyngeal carcinoma.
Conclusions
High prevalence of HPV infection in studied population strengthens the idea of HPV role in etiology of laryngeal cancer. Similarly, the low expression of PTEN and higher expression of p16INK4 expression signature was observed in the present study but did not yield any correlation with prognosis and other clinical parameters. The association of EGFR over expression with lymph node status and advanced stage of the disease supports the role that EGFR plays in tumor metastasis and invasion in laryngeal carcinoma patients. Therefore, the use of anti-EGFR targeted therapy in cases of laryngeal carcinoma may improve the prognosis of these patients.
Acknowledgments
The authors would like to thank Mr. Mohammed H. Al-Shamlan for his technical support.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia (IRB number: IRB-2014-08-044) and written informed consent was obtained from all patients. The study outcomes don’t affect the future management of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin 2017;67:31-50. [Crossref] [PubMed]
- Shin JY, Truong MT. Racial disparities in laryngeal cancer treatment and outcome: A population-based analysis of 24,069 patients. Laryngoscope 2015;125:1667-74. [Crossref] [PubMed]
- DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin 2013;63:151-66. [Crossref] [PubMed]
- Lubin JH, Purdue M, Kelsey K, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol 2009;170:937-47. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- O'Rorke MA, Ellison MV, Murray LJ, et al. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol 2012;48:1191-201. [Crossref] [PubMed]
- Brandstorp-Boesen J, Sørum Falk R, Folkvard Evensen J, et al. Risk of Recurrence in Laryngeal Cancer. PLoS One 2016;11:e0164068. [Crossref] [PubMed]
- Sahoo R, Chittibabu V, Patil G, et al. Relationship between molecular markers and treatment response in a retrospective cohort of Indian patients with primary carcinoma of the larynx. Oral Oncol 2009;45:e216-21. [Crossref] [PubMed]
- Stewart BW, Wild CP. World Cancer Report 2014. Lyon: International Agency for Research on Cancer, 2014.
- Ozanne B, Richards CS, Hendler F, et al. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol 1986;149:9-14. [Crossref] [PubMed]
- Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol 2013;49:287-92. [Crossref] [PubMed]
- Yewale C, Baradia D, Vhora I, et al. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials 2013;34:8690-707. [Crossref] [PubMed]
- Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 2009;4:127-50. [Crossref] [PubMed]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 2012;13:283-96. [Crossref] [PubMed]
- Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 2018;19:547-62. [Crossref] [PubMed]
- Romagosa C, Simonetti S, López-Vicente L, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011;30:2087-97. [Crossref] [PubMed]
- Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry 2011;50:5566-82. [Crossref] [PubMed]
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9-22. [Crossref] [PubMed]
- Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med 2007;357:2552-61. [Crossref] [PubMed]
- Krikelis D, Kotoula V, Bobos M, et al. Protein and mRNA expression of notch pathway components in operable tumors of patients with laryngeal cancer. Anticancer Res 2014;34:6495-503. [PubMed]
- de Miguel-Luken MJ, Chaves-Conde M, Quintana B, et al. Phosphorylation of gH2AX as a novel prognostic biomarker for laryngoesophageal dysfunction-free survival. Oncotarget 2016;7:31723-37. [Crossref] [PubMed]
- Lu B, Li J, Gao Q, et al. Laryngeal cancer risk and common single nucleotide polymorphisms in nucleotide excision repair pathway genes ERCC1, ERCC2, ERCC3, ERCC4, ERCC5 and XPA. Gene 2014;542:64-8. [Crossref] [PubMed]
- O'Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015;66:311-28. [Crossref] [PubMed]
- Al-Amri AM, Vatte C, Cyrus C, et al. Novel mutations of PIK3CA gene in head and neck squamous cell carcinoma. Cancer Biomark 2016;16:377-83. [Crossref] [PubMed]
- Vatte C, Al Amri AM, Cyrus C, et al. Tyrosine kinase domain mutations of
EGFR gene in head and neck squamous cell carcinoma. Onco Targets Ther 2017;10:1527-33. [Crossref] [PubMed] - Kontić M, Milovanović J, Čolović Z, et al. Epidermal growth factor receptor (EGFR) expression in patients with laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 2015;272:401-5. [Crossref] [PubMed]
- Jiang M, Zhang H, Xiao H, et al. High expression of c-Met and EGFR is associated with poor survival of patients with glottic laryngeal squamous cell carcinoma. Oncol Lett 2018;15:931-39. [PubMed]
- Maiti GP, Mondal P, Mukherjee N, et al. Overexpression of EGFR in head and neck squamous cell carcinoma is associated with inactivation of SH3GL2 and CDC25A genes. PLoS One 2013;8:e63440. [Crossref] [PubMed]
- Ding D. Studies on the expression and relationship between MMP-9 and EGFR in laryngeal squamous cell carcinoma. Biomedical Research 2017;S699-702.
- Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck 2016;38:E1625-38. [Crossref] [PubMed]
- Mastronikolis NS, Tsiambas E, Papadas TA, et al. Deregulation of PTEN Expression in Laryngeal Squamous Cell Carcinoma Based on Tissue Microarray Digital Analysis. Anticancer Res 2017;37:5521-4. [PubMed]
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12-9. [Crossref] [PubMed]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342-50. [Crossref] [PubMed]
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92:709-20. [Crossref] [PubMed]
- Hobbs CG, Sterne JA, Bailey M, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 2006;31:259-66. [Crossref] [PubMed]
- Ma XL, Ueno K, Pan ZM, et al. Human papillomavirus DNA sequences and p53 over-expression in laryngeal squamous cell carcinomas in Northeast China. J Med Virol 1998;54:186-91. [Crossref] [PubMed]
- Fouret P, Monceaux G, Temam S, et al. Human papillomavirus in head and neck squamous cell carcinomas in nonsmokers. Arch Otolaryngol Head Neck Surg 1997;123:513-6. [Crossref] [PubMed]
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467-75. [Crossref] [PubMed]
- Li X, Gao L, Li H, et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis 2013;207:479-88. [Crossref] [PubMed]
- Robinson M, Sloan P, Shaw R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol 2010;46:492-6. [Crossref] [PubMed]


