The role of proton beam therapy in the management of elderly breast cancer patients
Introduction
In 2019, an estimated 268,600 women will be diagnosed with breast cancer in the United States making breast cancer responsible for 30% of new female cancer diagnoses (1). Breast cancer prevalence increases dramatically with age such that an American woman over age 70 has a one out of fifteen probability of receiving a new breast cancer diagnosis (1). Elderly women tend to present with more favorable breast tumors: smaller tumor sizes, higher steroid receptor expression, lower rates of proliferation, less poorly differentiated and fewer nodal metastases (2-4). Despite their more favorable tumor characteristics, breast cancer carries a worse prognosis in this population regardless of cancer stage (5,6). Co-morbid conditions play a role in the decrease in overall survival (OS) with advancing age (7-9), but breast cancer disease-specific survival (DFS) is also worse in elderly patients (5). This may possibly be due to an inability to tolerate aggressive therapy for locally advanced disease.
Adjuvant radiation therapy (RT) is a standard component of the treatment course for non-metastatic breast cancer (10). Most patients with early stage (stage I–II) breast cancer are candidates for breast-conserving therapy, which consists of conservative surgery (e.g., lumpectomy) followed by RT. The benefit of preventing local recurrence with adjuvant RT following breast-conserving therapy has been demonstrated in multiple large randomized trials in women with stage I-II disease (11-21). Although the addition of adjuvant RT following breast-conserving surgery did not provide an OS or distant DFS benefit in these trials, breast-conserving surgery with adjuvant RT provides similar local recurrence, DFS and OS to treatment with mastectomy (19). Furthermore, a meta-analysis of 17 randomized trials found a DFS and cancer-specific survival benefit of adjuvant RT following breast-conserving surgery in patients with T1/T2 tumors regardless of nodal status (22). Therefore, breast-conserving therapy is a safe and effective alternative to mastectomy for most women with stage I–II breast cancer.
Patients with locally advanced breast cancer often undergo neoadjuvant chemotherapy to facilitate downstaging for possible breast-conserving therapy (23-25). Patients with multiple risk factors for locoregional recurrence (multifocal residual disease, lymphovascular invasion, clinical N2-N3 disease or residual pathological tumor size greater than 2 cm) benefit from a multimodality approach with neoadjuvant chemotherapy, mastectomy and postmastectomy radiation therapy (PMRT) (26). Clinical trials have found PMRT to reduce local-regional recurrence and improve cancer-specific survival and OS in patients with high-risk breast cancer who receive adjuvant chemotherapy (27-29). A randomized clinical trial has not evaluated PMRT in patients who received neoadjuvant chemotherapy. However, an analysis of patients on clinical protocols at MD Anderson demonstrated a local regional recurrence and cancer-specific survival benefit from PMRT in patients with high-risk breast cancer who received neoadjuvant chemotherapy (30). Thus, PMRT is part of the current guidelines for patients at high risk of locoregional recurrence (10).
Several techniques have been developed to deliver radiation dose for breast cancer: whole-breast irradiation (WBI), accelerated partial-breast irradiation (APBI) and chest wall irradiation. RT for breast cancer has traditionally been delivered with photons, but over the last two decades clinical trials have explored the use of proton beam radiation therapy (PBRT) because of its potential to deliver improved target dose coverage and normal tissue sparing (31-38). In this review, we will discuss adjuvant photon therapy in the elderly population before discussing PBRT for breast cancer and the role of PBRT in elderly breast cancer patients.
The role of RT for elderly breast cancer patients
Similar to the general population, adjuvant RT provides elderly patients with early stage (stage I–II) breast cancer a local recurrence benefit without evidence of improvement in DFS or OS. A sub-analysis of patients over age 65 in a randomized clinical trial of women with breast carcinoma less than 2.5 cm in size, with no restriction on hormonal status, treated with breast-conserving surgery and axillary lymph node dissection found no ipsilateral breast recurrence benefit of adjuvant RT (39). Two subsequent, large clinical trials randomized elderly women (age ≥65 or age ≥70) with low-risk breast cancer (tumor size <2–3 cm, hormone receptor positive, nodal negative) treated with breast-conserving surgery and adjuvant hormonal therapy to WBI or no WBI. Both trials found a small, but significant, decrease in ipsilateral breast recurrence without a difference in DFS or OS (14,15,18). Given this data, elderly women over age 65 with low-risk breast cancer (T1N0M0, hormonal positive) treated with breast conserving surgery and adjuvant hormonal therapy may reasonably omit adjuvant RT. However, since the publication of these trials a large proportion of elderly patients with low-risk breast cancer treated with breast conserving surgery and adjuvant hormonal therapy continue to receive adjuvant RT (40-42).
Less-selective clinical trials have found adjuvant RT to benefit elderly patients with early breast cancer. The aforementioned meta-analysis of 17 randomized trials of patients with T1–T2 breast cancer included patients who were nodal positive and estrogen receptor (ER) negative (22). When the patients were stratified by age, patients age 60–69 and above age 70 had a similar relative reduction in DFS with adjuvant RT as the entire cohort, albeit with a smaller absolute reduction.
The benefits of PMRT for elderly patients with advanced breast cancer are not as well studied as breast-conserving therapy. The Danish Breast Cancer Cooperative Group 82c (DBCG 82c) trial found PMRT to improve survival in postmenopausal women with advanced breast cancer treated with adjuvant hormonal therapy (27). However, this trial only included postmenopausal women under age 70. An analysis of women with advanced breast cancer treated with mastectomy without PMRT found similar locoregional recurrence rates between women age 50–69 and women age 70 years and older (43), suggesting that patients over age 70 would receive similar benefit from PMRT in preventing locoregional recurrence as patients under age 70. Using the Surveillance, Epidemiology and End Results (SEER) database, Smith et al. found that PMRT improved OS in patients over age 70 with high risk features (tumor greater than 5 cm and/or more than 4 positive lymph nodes) (44).
Treatment toxicity is an important consideration in the elderly population. Elderly patients experience worse toxicity secondary to radiation and systemic therapy, resulting in decreased treatment compliance (45). Although elderly patients treated with hypo-fractionated WBI experience slightly worse toxicity compared to their younger counterparts, it may be beneficial in patients unable to sustain weeks of daily treatment (46-48). Furthermore, the addition of a tumor bed boost is associated with worse toxicity without survival benefit in elderly breast cancer patients (48). Two trials evaluating external beam APBI in elderly patients with early-stage breast cancer documented acceptable toxicity in this population (49,50).
PBRT for breast cancer
PBRT may be used in place of photons for breast cancer and can be used for WBI, APBI or PMRT. Dosimetric studies have demonstrated the ability of PBRT to provide WBI with superior target coverage with improved organ-at-risk sparing (33-35,37,51). When compared to IMRT delivered with photons, PBRT improves target dose homogeneity, while minimizing cardiac and pulmonary dose (31,32,35,38). These dosimetric improvements are also found with regional node coverage delivered with PBRT (36,51). Enhanced inspiratory gating provides further dose improvement with PBRT compared with photon-delivered WBI (51). Northwestern University reported their experience using PBRT for WBI with comprehensive nodal irradiation in 27 breast cancer patients following lumpectomy (52). All patients experienced dermatitis, 56% experienced grade 2 dermatitis and 7% experienced grade 3 dermatitis. Grade 1 esophagitis occurred in 33% of patients and grade 2 esophagitis occurred in 30% of patients, but there was no grade 3 esophagitis. Although there were no reported cases of grade 3 breast pain, over half of the patients experienced grade 2 breast pain. Despite these results, a prospective clinical trial has not evaluated the safety or efficacy of WBI delivered with PBRT.
As with WBI, a number of dosimetric analyses comparing APBI for early-stage breast cancer delivered with protons versus photons found PBRT to deliver superior target coverage with reduced cardiopulmonary dose (53-57). Unlike WBI, there have been several prospective clinical trials evaluating the utility of PBRT as a method for delivering APBI. Massachusetts General Hospital was the first to report on their experience using PBRT for APBI (58). Their treatment schedule consisted of 8 fractions of 4 Gy [relative biological equivalent (RBE)] delivered twice daily to 20 patients with stage I breast cancer. Patients had acceptable cosmetic outcomes, but experienced significant acute skin toxicity. Over three quarters of patients developed moderate to severe skin color changes at 3 to 4 weeks and 22% developed moderate to severe moist desquamation. One patient developed a rib fracture and three had rib tenderness. Long-term follow-up found similar survival rates and non-cutaneous toxicity between patients treated with APBI with photons versus protons (59). However, patients treated with PBRT had worse skin toxicity and cosmetic outcomes compared to patients treated with photons.
Loma Linda subsequently performed a larger 100 patient trial using PBRT for APBI in patients with tumors less than 3 cm in size and nodal negative disease (60,61). They delivered a dose of 40 Gy (RBE) in ten fractions over two weeks using 2–4 beams. Their population had a mean age of 63, with a range of 41–83. They reported a 5-year DFS of 94% and OS of 95%. Although 62% of patients suffered from grade 1–2 acute skin toxicity, there was no grade 3 or higher acute skin toxicity. One patient suffered from fat necrosis and 7% suffered from grade 1 telangiectasia. There were no reported rib fractures, cardiac events, breast infections or pneumonitis. Furthermore, 90% of patients reported good or excellent cosmetic outcome. In contrast to the results reported at Massachusetts General Hospital, a cross-sectional analysis of patients who were treated with APBI with protons at Loma Linda reported better cosmesis, less breast pain and reduced fatigue at 6.5 years post-treatment compared to women treated with photon-delivered WBI (62).
A Korean trial using APBI delivered with PBRT enrolled 30 patients with a median age of 48 that had tumors equal or less than 3 cm in size (63). A dose of 30 Gy (RBE) was delivered in six fractions over five consecutive days. Half the patients were treated with a single field while two proton beams were used for the remaining 15 patients. With a median follow-up of 59 months, there were no disease recurrences or deaths. One patient experienced wet desquamation and two patients suffered rib fractures. At one-year post-treatment 30% of patients suffered from mild to moderate induration. The toxicity profile was worse in the patients treated with a single field versus multiple fields.
MD Anderson reported their short-term toxicities of 43 patients with stage I breast cancer treated with APBI by protons (64). Only 16% of patients had dry desquamation and only one developed wet desquamation. A six-month mammographic evaluation found that 40% of patients had skin thickening, 14% had a seroma/hematoma, 2% developed fat necrosis and 26% had retraction or asymmetry present.
Like APBI, PBRT has also been shown to be a viable treatment modality for PMRT. Dosimetric studies comparing protons to photons for PMRT have found PBRT to provide better target homogeneity, while limiting dose to the heart and lungs (35,65-68). These studies primarily looked at left-sided breast cancer. The target dosing benefit of protons has been demonstrated with both 3D-conformal, passively scattered proton beam radiation and intensity modulated proton therapy (IMPT). PBRT can be used for PMRT in patients following breast reconstruction or insertion of tissue expanders (67,69).
The first clinical trial utilizing PBRT for PMRT enrolled twelve patients at Massachusetts General Hospital with locally-advanced breast cancer (70). Patients were treated with passively scattered proton fields with a dose of 50.4 Gy (RBE) to the chest wall and 45–50.4 Gy (RBE) to the nodal targets at risk. The patient ages ranged from 31 to 68 and 11 out of 12 patients had left-sided breast cancer. There were 9 patients with grade 2 acute skin toxicity, with the remaining three patients developing grade 1 skin toxicity during treatment. There was no documented acute pulmonary or cardiac toxicity.
Memorial Sloan Kettering reported on their experience treating 42 patients with PMRT delivered with protons using 3D conformal uniform scanning (71,72). The patients’ age ranged from 21 to 86, with a median of 46.5. The patient’s chest wall and regional nodes were treated to a dose of 50.4 Gy (RBE). After a median follow-up of 35 months there was one locoregional failure, six distant failures and one death in a patient with metastatic disease. 74% of patients developed grade 2 acute dermatitis, with the remaining 26% having grade 1 dermatitis. There was moist desquamation in 21% of patients and half of patients developed grade 1 or 2 acute esophagitis. Over a quarter of patients who underwent immediate reconstruction had reconstruction complications. There was one case of pneumonitis and no rib fractures.
Northwestern was the first to document their experience using pencil beam scanning (PBS) for breast cancer (52). Patients were selected with unacceptable treatment plans when planned with photon RT. They treated 21 patients after lumpectomy or mastectomy with adjuvant PBRT using PBS. One patient developed grade 3 dermatitis, 76% had grade 2 dermatitis and the remaining 19% had grade 1 dermatitis. 71% of patients developed grade 1 or 2 esophagitis and 67% had grade 2 chest wall or breast pain.
Although a National Cancer Database (NCDB) analysis found no difference in survival between patients with non-metastatic breast cancer who received adjuvant RT with protons versus with photons or electrons (73), there have been no prospective trials published comparing survival between breast cancer patients receiving PBRT and photons. RADCOMP trial is a pragmatic prospective randomized clinical trial of patients with locally advanced (stage II and III) breast cancer, randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. This study has more than 20 proton centers in the US and expects to enroll about 1,800 patients with stage II–III right and left breast cancer. Patients will be treated with either PBRT or photons for 45 to 50 Gy to the breast/chest wall and comprehensive regional lymphatics including the internal mammary chain.
PBRT in the elderly breast cancer population
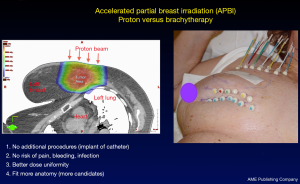
PBRT offers elderly breast cancer patients similar potential for disease control with reduced toxicity as the general population. In elderly patients with early stage and low-risk breast cancer, APBI delivered with protons can provide excellent cosmesis and good disease control (60,61). Dosimetric analysis of APBI by protons demonstrates improved target coverage and homogeneity while reducing cardiopulmonary toxicity (Figure 1) (56,57). Compared to APBI delivered with brachytherapy, proton APBI provides patients the ability to avoid additional procedures required with brachytherapy (Figure 2). This reduces the risk of pain, bleeding and infection conferred by catheter implantation during brachytherapy. PBRT also has fewer anatomical restrictions allowing more elderly patients to receive APBI.
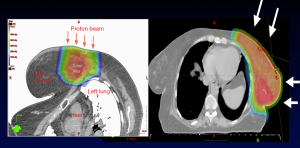
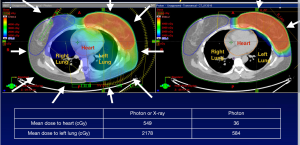
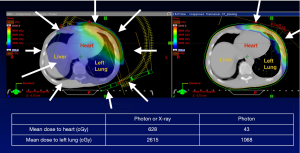
In elderly breast cancer patients not eligible for APBI, WBI delivered with protons reduces radiation dose to the lung and heart (34,37,51). The reduction in cardiac toxicity is particularly pronounced in women with left-sided breast cancer (Table 1, Figures 3,4) (74). Breast cancer RT doses to the heart may result in increased risk of cardiovascular disease and cardiac mortality (75).
Table 1
| Variable | Mean heart dose (Gy) (with IMN) | Mean heart dose (Gy) (without IMN) |
|---|---|---|
| All modalities | 4.2 | 8.0 |
| Photons | 1.2 (0.8–1.7) | 9.2 (1.9–21.0) |
| Photons (IMRT) | 5.6 (0.1–23.0) | |
| Photons (DIBH) | 1.3 (0.4–2.5) | |
| Protons | 0.5 (0.1–0.8) | 2.6 (1.0–6.0) |
DIBH, deep inspiration breath hold; IMRT, intensity-modulated radiation therapy; IMN, internal mammary lymph nodes.
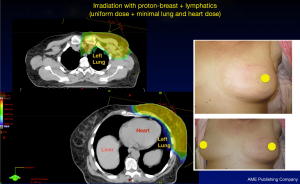
PBRT may be used in elderly patients with more advanced breast cancer for PMRT (71,72). PBRT provides superior cardiopulmonary sparing in women receiving PMRT compared to photons (Figure 5) (36,52,65,66,68). PBRT also offers superior chest wall coverage and target homogeneity. For patients requiring nodal irradiation, PBRT also provides better nodal coverage compared to photons (36,65).
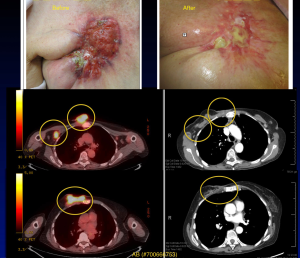
Other indications for PBRT in the elderly breast cancer population include definitive radiation and palliative care. In elderly breast cancer patients who are not surgical candidates, limited dosimetric analyses have found PBRT to be a viable option for definitive therapy (76). PBRT may also be used for patients treated with palliative intent (Figure 6).
There are important considerations to be made when treating elderly patients with PBRT (77). As a group, elderly breast cancer patients have worse OS due to their comorbidities (8,9). One study found that patients with a Charlson Comorbidity Index of 1–2 had worse OS following breast-conserving therapy compared to patients with a score of 0 (8). Screening tools, such as the G-8 and Timed Up and Go, have been developed and validated in cancer patients to predict mortality in the elderly population (78-80). These tests may help to identify elderly patients who are suitable candidates for PBRT, as well as determine the patients who will require cognitive or social support to make it through their therapy course. These tests have limitations as they do not predict acute toxicity or RT treatment compliance (45).
Conclusions
PBRT is a safe and viable adjuvant treatment option for elderly breast cancer patients with both early-stage disease receiving breast-conserving therapy and those with more locally advanced breast cancer requiring chest wall irradiation following mastectomy. PBRT provides superior target coverage while reducing dose to the organs at risk. PBRT can also be used for palliative therapy. However, further research is necessary to fully understand the risks and benefits of PBRT in elderly breast cancer patients. In the future, newer methods of proton beam delivery such as PBS may provide further benefit for this patient population.
Acknowledgments
Funding: This work was supported by funds provided by the University of Miami Miller School of Medicine/Sylvester Comprehensive Cancer Center Department of Radiation Oncology.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.40). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. HG serves as an unpaid Co-Editor-in-Chief of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Malik MK, Tartter PI, Belfer R. Undertreated breast cancer in the elderly. J Cancer Epidemiol 2013;2013:893104. [Crossref] [PubMed]
- Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 2000;92:550-6. [Crossref] [PubMed]
- Singh R, Hellman S, Heimann R. The natural history of breast carcinoma in the elderly: implications for screening and treatment. Cancer 2004;100:1807-13. [Crossref] [PubMed]
- van de Water W, Markopoulos C, van de Velde CJ, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA 2012;307:590-7. [Crossref] [PubMed]
- Brandt J, Garne JP, Tengrup I, et al. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol 2015;13:33. [Crossref] [PubMed]
- Patnaik JL, Byers T, Diguiseppi C, et al. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 2011;103:1101-11. [Crossref] [PubMed]
- Harris EE, Hwang WT, Urtishak SL, et al. The impact of comorbidities on outcomes for elderly women treated with breast-conservation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys 2008;70:1453-9. [Crossref] [PubMed]
- Siegelmann-Danieli N, Khandelwal V, Wood GC, et al. Breast cancer in elderly women: outcome as affected by age, tumor features, comorbidities, and treatment approach. Clin Breast Cancer 2006;7:59-66. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 4.2018) Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002;20:4141-9. [Crossref] [PubMed]
- Winzer KJ, Sauerbrei W, Braun M, et al. Radiation therapy and tamoxifen after breast-conserving surgery: updated results of a 2 x 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer 2010;46:95-101. [Crossref] [PubMed]
- Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004;351:963-70. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004;351:971-7. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294-302. [Crossref] [PubMed]
- Tinterri C, Gatzemeier W, Zanini V, et al. Conservative surgery with and without radiotherapy in elderly patients with early-stage breast cancer: a prospective randomised multicentre trial. Breast 2009;18:373-7. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Ford HT, Coombes RC, Gazet JC, et al. Long-term follow-up of a randomised trial designed to determine the need for irradiation following conservative surgery for the treatment of invasive breast cancer. Ann Oncol 2006;17:401-8. [Crossref] [PubMed]
- Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol 1999;17:2326-33. [Crossref] [PubMed]
- Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 2004;22:2303-12. [Crossref] [PubMed]
- van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37. [Crossref] [PubMed]
- Akay CL, Meric-Bernstam F, Hunt KK, et al. Evaluation of the MD Anderson Prognostic Index for local-regional recurrence after breast conserving therapy in patients receiving neoadjuvant chemotherapy. Ann Surg Oncol 2012;19:901-7. [Crossref] [PubMed]
- Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641-8. [Crossref] [PubMed]
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949-55. [Crossref] [PubMed]
- Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956-62. [Crossref] [PubMed]
- Huang EH, Tucker SL, Strom EA, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol 2004;22:4691-9. [Crossref] [PubMed]
- Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol 2002;62:137-45. [Crossref] [PubMed]
- Lomax AJ, Cella L, Weber D, et al. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys 2003;55:785-92. [Crossref] [PubMed]
- Bush DA, Slater JD, Garberoglio C, et al. A technique of partial breast irradiation utilizing proton beam radiotherapy: comparison with conformal x-ray therapy. Cancer J 2007;13:114-8. [Crossref] [PubMed]
- Mast ME, Vredeveld EJ, Credoe HM, et al. Whole breast proton irradiation for maximal reduction of heart dose in breast cancer patients. Breast Cancer Res Treat 2014;148:33-9. [Crossref] [PubMed]
- Ares C, Khan S, Macartain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys 2010;76:685-97. [Crossref] [PubMed]
- Bradley JA, Dagan R, Ho MW, et al. Initial Report of a Prospective Dosimetric and Clinical Feasibility Trial Demonstrates the Potential of Protons to Increase the Therapeutic Ratio in Breast Cancer Compared With Photons. Int J Radiat Oncol Biol Phys 2016;95:411-21. [Crossref] [PubMed]
- Xu N, Ho MW, Li Z, et al. Can proton therapy improve the therapeutic ratio in breast cancer patients at risk for nodal disease? Am J Clin Oncol 2014;37:568-74. [Crossref] [PubMed]
- Lin LL, Vennarini S, Dimofte A, et al. Proton beam versus photon beam dose to the heart and left anterior descending artery for left-sided breast cancer. Acta Oncol 2015;54:1032-9. [Crossref] [PubMed]
- Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol 2001;12:997-1003. [Crossref] [PubMed]
- Rutter CE, Lester-Coll NH, Mancini BR, et al. The evolving role of adjuvant radiotherapy for elderly women with early-stage breast cancer. Cancer 2015;121:2331-40. [Crossref] [PubMed]
- Palta M, Palta P, Bhavsar NA, et al. The use of adjuvant radiotherapy in elderly patients with early-stage breast cancer: changes in practice patterns after publication of Cancer and Leukemia Group B 9343. Cancer 2015;121:188-93. [Crossref] [PubMed]
- Luu C, Goldstein L, Goldner B, et al. Trends in radiotherapy after breast-conserving surgery in elderly patients with early-stage breast cancer. Ann Surg Oncol 2013;20:3266-73. [Crossref] [PubMed]
- Truong PT, Lee J, Kader HA, et al. Locoregional recurrence risks in elderly breast cancer patients treated with mastectomy without adjuvant radiotherapy. Eur J Cancer 2005;41:1267-77. [Crossref] [PubMed]
- Smith BD, Haffty BG, Hurria A, et al. Postmastectomy radiation and survival in older women with breast cancer. J Clin Oncol 2006;24:4901-7. [Crossref] [PubMed]
- Middelburg JG, Mast ME, de Kroon M, et al. Timed Get Up and Go Test and Geriatric 8 Scores and the Association With (Chemo-)Radiation Therapy Noncompliance and Acute Toxicity in Elderly Cancer Patients. Int J Radiat Oncol Biol Phys 2017;98:843-9. [Crossref] [PubMed]
- Ortholan C, Hannoun-Lévi JM, Ferrero JM, et al. Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys 2005;61:154-62. [Crossref] [PubMed]
- Giugliano FM, Falivene S, Esposito E, et al. Short-course radiotherapy in elderly women with breast cancer: Comparison by age, comorbidity index and toxicity. Int J Surg 2016;33:S92-6. [Crossref] [PubMed]
- De Santis MC, Bonfantini F, Di Salvo F, et al. Hypofractionated Whole-Breast Irradiation With or Without Boost in Elderly Patients: Clinical Evaluation of an Italian Experience. Clin Breast Cancer 2018;18:e1059-66. [Crossref] [PubMed]
- Sayan M, Wilson K, Nelson C, et al. A novel schedule of accelerated partial breast radiation using intensity-modulated radiation therapy in elderly patients: survival and toxicity analysis of a prospective clinical trial. Radiat Oncol J 2017;35:32-8. [Crossref] [PubMed]
- Jacobs DHM, Speijer G, Petoukhova AL, et al. Acute toxicity of intraoperative radiotherapy and external beam-accelerated partial breast irradiation in elderly breast cancer patients. Breast Cancer Res Treat 2018;169:549-59. [Crossref] [PubMed]
- Flejmer AM, Edvardsson A, Dohlmar F, et al. Respiratory gating for proton beam scanning versus photon 3D-CRT for breast cancer radiotherapy. Acta Oncol 2016;55:577-83. [Crossref] [PubMed]
- Verma V, Iftekaruddin Z, Badar N, et al. Proton beam radiotherapy as part of comprehensive regional nodal irradiation for locally advanced breast cancer. Radiother Oncol 2017;123:294-8. [Crossref] [PubMed]
- Wang X, Zhang X, Li X, et al. Accelerated partial-breast irradiation using intensity-modulated proton radiotherapy: do uncertainties outweigh potential benefits? Br J Radiol 2013;86:20130176. [Crossref] [PubMed]
- Taghian AG, Kozak KR, Katz A, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys 2006;65:1404-10. [Crossref] [PubMed]
- Kozak KR, Katz A, Adams J, et al. Dosimetric comparison of proton and photon three-dimensional, conformal, external beam accelerated partial breast irradiation techniques. Int J Radiat Oncol Biol Phys 2006;65:1572-8. [Crossref] [PubMed]
- Moon SH, Shin KH, Kim TH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol 2009;90:66-73. [Crossref] [PubMed]
- Wang X, Amos RA, Zhang X, et al. External-beam accelerated partial breast irradiation using multiple proton beam configurations. Int J Radiat Oncol Biol Phys 2011;80:1464-72. [Crossref] [PubMed]
- Kozak KR, Smith BL, Adams J, et al. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys 2006;66:691-8. [Crossref] [PubMed]
- Galland-Girodet S, Pashtan I, MacDonald SM, et al. Long-term cosmetic outcomes and toxicities of proton beam therapy compared with photon-based 3-dimensional conformal accelerated partial-breast irradiation: a phase 1 trial. Int J Radiat Oncol Biol Phys 2014;90:493-500. [Crossref] [PubMed]
- Bush DA, Slater JD, Garberoglio C, et al. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer 2011;11:241-5. [Crossref] [PubMed]
- Bush DA, Do S, Lum S, et al. Partial breast radiation therapy with proton beam: 5-year results with cosmetic outcomes. Int J Radiat Oncol Biol Phys 2014;90:501-5. [Crossref] [PubMed]
- Teichman SL, Do S, Lum S, et al. Improved long-term patient-reported health and well-being outcomes of early-stage breast cancer treated with partial breast proton therapy. Cancer Med 2018;7:6064-76. [Crossref] [PubMed]
- Chang JH, Lee NK, Kim JY, et al. Phase II trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol 2013;108:209-14. [Crossref] [PubMed]
- Ovalle V, Strom EA, Shaitelman S, et al. Proton Partial Breast Irradiation: Detailed Description of Acute Clinico-Radiologic Effects. Cancers (Basel) 2018; [Crossref] [PubMed]
- MacDonald SM, Jimenez R, Paetzold P, et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol 2013;8:71. [Crossref] [PubMed]
- Patel SA, Lu HM, Nyamwanda JA, et al. Postmastectomy radiation therapy technique and cardiopulmonary sparing: A dosimetric comparative analysis between photons and protons with free breathing versus deep inspiration breath hold. Pract Radiat Oncol 2017;7:e377-84. [Crossref] [PubMed]
- Jimenez RB, Goma C, Nyamwanda J, et al. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol 2013;107:213-7. [Crossref] [PubMed]
- Hernandez M, Zhang R, Sanders M, et al. A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with post-mastectomy radiotherapy. J Proton Ther 2015. doi:
10.14319/jpt.11.9 . - Mutter RW, Remmes NB, Kahila MM, et al. Initial clinical experience of postmastectomy intensity modulated proton therapy in patients with breast expanders with metallic ports. Pract Radiat Oncol 2017;7:e243-52. [Crossref] [PubMed]
- MacDonald SM, Patel SA, Hickey S, et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013;86:484-90. [Crossref] [PubMed]
- Luo L, Cuaron J, Braunstein L, et al. Early outcomes of breast cancer patients treated with post-mastectomy uniform scanning proton therapy. Radiother Oncol 2019;132:250-6. [Crossref] [PubMed]
- Cuaron JJ, Chon B, Tsai H, et al. Early toxicity in patients treated with postoperative proton therapy for locally advanced breast cancer. Int J Radiat Oncol Biol Phys 2015;92:284-91. [Crossref] [PubMed]
- Chowdhary M, Lee A, Gao S, et al. Is Proton Therapy a "Pro" for Breast Cancer? A Comparison of Proton vs. Non-proton Radiotherapy Using the National Cancer Database. Front Oncol 2019;8:678. [Crossref] [PubMed]
- Taylor CW, Wang Z, Macaulay E, et al. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int J Radiat Oncol Biol Phys 2015;93:845-53. [Crossref] [PubMed]
- Cheng YJ, Nie XY, Ji CC, et al. Long-Term Cardiovascular Risk After Radiotherapy in Women With Breast Cancer. J Am Heart Assoc 2017; [Crossref] [PubMed]
- Lischalk JW, Chen H, Repka MC, et al. Definitive hypofractionated radiation therapy for early stage breast cancer: Dosimetric feasibility of stereotactic ablative radiotherapy and proton beam therapy for intact breast tumors. Adv Radiat Oncol 2018;3:447-57. [Crossref] [PubMed]
- Thariat J, Sio T, Blanchard P, et al. Using Proton Beam Therapy in the Elderly Population: A Snapshot of Current Perception and Practice. Int J Radiat Oncol Biol Phys 2017;98:840-2. [Crossref] [PubMed]
- Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166-72. [Crossref] [PubMed]
- Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8. [Crossref] [PubMed]
- Ferrat E, Paillaud E, Laurent M, et al. Predictors of 1-Year Mortality in a Prospective Cohort of Elderly Patients With Cancer. J Gerontol A Biol Sci Med Sci 2015;70:1148-55. [Crossref] [PubMed]