
Fluid shear stress-induced IL-8/CXCR signaling in human ovarian cancer cells
Introduction
Ovarian carcinoma is one of the most common and lethal gynecological malignancy, expected to cause over 14,000 deaths in American in 2018, with a 5-year survival rate that can be as low as 30% when diagnosed after cancer (1). The development of ovarian cancer begins at the ovary and spreads mainly in the peritoneal cavity (2,3). During metastasis, ovarian cancer cells are shed from the primary tumor and disseminate throughout the peritoneal cavity (4). These disseminating ovarian cancer cells can adhere to the organs in the peritoneal cavity to initiate secondary tumor foci, which worsens the prognosis of patients (5,6). The ovarian cancer cells in the peritoneal cavity are exposure to fluid motion, and this mechanical microenvironment is considered to be a prominent factor affecting the spread of ovarian cancer cells (7).
The physiologic environment is determined by chemical and physical factors which direct influence tissue function and contribute to diseases such as cancer. Physical forces have a crucial role in tumor progression and cancer treatment, and different biomechanical behaviors behind the tumor microenvironment can facilitate tumorigenesis and tumor progression (8). Mechanical stimuli such as fluid shear stresses (FSS) were suggested to affect many cellular processes in cancer cell such as cancer cell morphology, proliferation, apoptosis, adhesion, migration, and invasion (9-11). While there have been studies to characterize the physical tumor microenvironment, the full impact of physical stimuli still is incompletely known. For ovarian cancer, with the application of microfluidic technology and mechanical measurement methods, developments in tumor fluid mechanics accelerated. Increasing evidence now shows that fluid shear stress is an essential factor affecting fluid mechanics, and its role in metastasis has received increasing attention. The magnitude of FSS in the peritoneal cavity is highly variable due to the different body size, adipose tissue volume and diaphragm movement, which is estimated to be around 0.5–1 dyne/cm2 (12,13). Therefore, low FSS (0.5, 1.5, or 2.0 dyne/cm2) were applied in this experiment to ovarian cancer cells to simulate the ovarian cancer fluid mechanics by using microfluidic channels.
Up to now, the existing study proved that fluid shear stress could stimulate the expression of cytoskeleton protein of ovarian cancer cells and promote peritoneal metastasis of ovarian cancers, while how fluid shear stress promotes the peritoneal metastasis of ovarian cancers cells remains unknown (7). Current evidence showed that chemokines were related to the fluid shear stress-activated biological pathway and involved in tumor metastasis. Interleukin-8 (IL-8) is a member of the chemokine family and is released from several cell types in response to an inflammatory stimulus (14). IL-8 could promote tumor cell migration and be related to angiogenesis, tumor progression, and metastasis in many kinds of carcinomas (15-18). Also, IL-8 could be a response to fluid shear stress. When the fluid shear stress was loaded, the IL-8 was upregulated in the nucleus pulposus cells and endothelial cell (19,20). Additionally, IL-8 receptor (CXCR1 and CXCR2) plays a key role in the migration of mechanosensitive cells in our previous study (21). Taken together, IL-8/CXCR signaling driven by fluid flow microenvironment may be thought to help invasion and migration behaviors of cancer cells away from primary tumors.
Accordingly, our investigations in this study focused on IL-8 and its receptors signal transduction process of human ovarian cancer cells under conditions of fluid shear flow. The IL-8 gene expression and protein production in human ovarian cancer cell line and its transcriptional activation were detected when the fluid shear stress was loaded. Overall, our investigations hope to reveal the mechano-chemical coupling mechanism of the response of ovarian cancer cell during exposed to fluid shear flow.
Methods
Cell cultures
Human ovarian cancer SKOV3 cells were obtained from Nanjing KeyGen Biotech. Inc. The SKOV3 cells were cultured inα-MEM medium (Hyclone, USA) containing 10% fetal bovine serum (FBS, Israel) supplemented with 100 units of penicillin (Sigma, USA) and 100 µg of streptomycin (Sigma, USA) per ml. The cells in their third or fourth passage were plated on a glass slide coated with 50 µg/mL fibronectin for use in the experiments. All cell cultures were kept in a humidified 5%/95% air incubator (Heraeus, Germany) at 37 °C.
Shear stress experiments
A flow chamber system was set up according to the design described previously15 with minor modifications. The fluid used to shear SKOV3 cells were free serum α-MEM. In brief, a 75 mm × 25 mm glass slide was seeded with SKOV3 cells, which were cultured until reaching a confluent monolayer. A silicone gasket was sandwiched between two polymethyl methacrylate plates to create a rectangular flow chamber (8.5 cm in length, 2.5 cm in width, and 0.03 cm in height) with inlet and outlet for exposing the cultured SKOV3 cells to shear stress. A steady, laminar flow across the flow chamber was generated by using a peristaltic pump (Cole-Parmer, USA). During the shear stress experiments, the flow system was kept at 37 °C in a constant temperature cabinet and equilibrated with 95% humidified air plus 5% CO2. In experiment about time, SKOV3 cells were exposed to low FSS (0.5, 1.5, or 2.0 dyne/cm2) for 1, 2, 3, 4, 5, 6, 8, 10, or 12 h, respectively. Normal static cultured SKOV3 cells were as a control with the same passage. In the experiment about intensity, SKOV3 cells were exposed to different FSS (0.5, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, or 5.0 dyne/cm2 respectively) for 1 or 2 h to determine IL-8 mRNA expression and 5 or 6 h to measure IL-8 protein production. Normal static cultured SKOV3 cells were also selected as a control.
RNA isolation and quantitative reversal transcription-polymerase chain reaction to measure the expression of IL-8 mRNA
Cells from static controls or from shear stress experiments were washed twice with ice-cold PBS, and total cellular RNA was isolated by TRIzol reagent (GIBCO-BRL, USA) according to the manufacturer’s manual. The RNA quality was ensured by gel visualization and spectrophotometric analysis (OD260/OD280). Quantitative RT-PCR assay involves LightCyclerTM technology. The experimental protocol was performed following the instruction manual of LightCycler-RNA Amplification Kit SYBR Green I (Roche Molecular Biochemicals, Germany). A 20 µL standard final reaction volume was prepared. All RT-PCRs were performed in a glass capillary (Roche Molecular Biochemicals, Germany), using 50 ng template total RNA. The sequence of the primers (forward primer: 5’-GCT AAA GAA CTT CGA TGT CAG TGC-3’, Reverse Primer: 5’-CTC AGC CCT CTT CAA AAA CTT CTC-3’) was used for the amplification of the IL-8 gene. Samples were placed into glass capillaries, capped, centrifuged for a few seconds in a micro-centrifuge using proper adapters, and then placed into the LightCyclerTM rotor. The thermal cycling conditions were as follows: 10 minutes at 55 °C for reverse transcription, 30 seconds at 95 °C for pre-denaturation, 42 cycles for 0 seconds at 95 °C for denaturation, 10 seconds at 55 °C for annealing, and 13 seconds at 72 °C for elongation. At the end of each cycle, the fluorescence emitted by the SYBR Green I was measured. For each RT-PCR product, apart from primer-dimers, a single narrow peak was obtained by melting curve analysis at the specific melting temperature. The RT-PCR products were subjected to analysis by direct sequencing (ABI/Prism 3700 Sequence System, USA) to confirm the efficiency of the melting curve analysis.
ELISA for quantification of IL-8 protein level
IL-8 protein levels in media were quantified using quantitative sandwich ELISA. 96-well round-bottom microtiter plates (Costa, Cambridge, USA) were coated with 200 mL/well of rabbit-anti-human IL-8 antibody (R&D, Minnesota, USA) diluted 1:2,000 in Voller’s buffer for 24 h at 4 °C. After three times washing in PBS-Tween, undiluted media and serial dilutions of standard human recombinant RIL-8 (Sigma, Missouri, USA) were incubated at room temperature for 90 min. Plates were rinsed 3 times with PBS-Tween followed by the addition of rabbit-anti-human IL-8 antibody (R&D, Minnesota, USA) diluted 1:2,000 in the washing solution. After 1 h incubation, dishes were washed 3 times, and peroxidase conjugated goat-anti-rabbit (Sigma, Missouri, USA) was added at a 1:2,000 dilution for 1 h incubation. The plates were then washed again, and orthophenyldiamine (Sigma, Missouri, USA) was dissolved in methanol (1 mg/mL) and diluted (10 ng/mL) in distilled water containing 0.01% H2O2 was added. The next reaction was stopped with 50 µL of 2 mol/L sulfuric acid, and plates were read at 492 nm at HTS 7000 Plus Bio Assay Reader (Perkin-Elmer, CT, USA).
Construction of IL-8 reporter gene PEGFP-IL8USCS and its transcriptional activation, and determination
PCR was used to amplify –102–+61 bp 5’ flanking promoter region of IL-8 gene (IL 8USCS) from genomic DNA of SKOV3 cells. The DNA fragment containing NF-κB binding site was cloned into pEGFP1, and the identity of recombinant plasmid pEGFP1-IL8USCS was confirmed by DNA sequencing. The SKOV3 cells were transfected with the pEGFP1-IL8USCS by using Dosper liposomal transfection reagent and selected by G418, then subjected to a shear stress of 1.5 dyne/cm2 for 3 hours. The green fluorescent protein expression was analyzed by flow cytometry. The experiment was repeated three times, and the results were shown as mean ± SD.
Immunofluorescence
The SKOV3 cells cultured on glass slides were fixed with 4% paraformaldehyde for 20 minutes and then blocked with 5% normal goat serum for 20 minutes. The cells were incubated in rabbit anti-human NF-κB p65 or CXCR-2 polyclonal antibody (1:100) for 1 hour, in biotinylate goat anti-rabbit immunoglobulins (1:300) for 45 minutes, and in FITC-conjugated streptavidin (1:100) for 15 minutes sequentially. 50% of glycerin alkaline buffer was used to seal the slides. Photographs were taken by using a fluorescent microscope or a laser confocal microscope (Bio-Rad, CA, USA).
Northern blot
The cDNA fragments of CXCR1 and CXCR2 (394 and 311 bp respectively) were used as probes. They were labeled using DIG DNA labeling and detection kit (Boechring Mannheim). The total RNAs were isolated from SKOV3 cells that were untreated or treated by fluid shear stress for 1 hour, as mentioned above. The RNAs were resolved by formaldehyde agarose gel electrophoresis at the constant voltage of 80 V. Northern blot was performed according to the protocol from The DIG System User’s Guide for Filter Hybridization distributed by Boehringer Mannheim. The Northern bands were visualized with NBT and BCIP.
Construction of truncated CXCR2 expression plasmid pcDNA3-mtCXCR2 and determination of its inhibitory effect on IL-8 reporter gene expression in SKOV3 cells
RT-PCR was used to amplify mtCXCR2 cDNA fragment (lacking 155 amino acids in the intracellular domain) from total RNA of SKOV3 cells. The cDNA fragment was cloned into pcDNA3 and the recombinant plasmid pcDNA3-mtCXCR2 by Dosper liposomal transfection regent, selected by G418, and followed by pEGFP1-IL8USCS co-transfection, and then stimulated with 1.5 dyne/cm2 shear stress for 3 hours. The green fluorescent protein expression was analyzed by flow cytometry.
Western blot analysis
Proteins were separated by 12% SDS-PAGE in Versatile Mini-Protein 3 Electrophoresis Cell (Bio-Rad, CA, USA) at the constant current of 30mA for about 2.5 h. Semi-dry transfer cell (Bio-Rad, CA, USA) was used to transfer proteins from SDS-PAGE gel onto the nitrocellulose membrane. Photope®HRP Western Detection Kit was used to determine phosphor and nonphospho-IkBa following the manufacturer’s instruction. The blot was sequentially incubated in 5% BSA blocking buffer at room temperature for 1 hour, in primary antibody (1:1,000) at 4 °C overnight, and in 10 mL of blocking buffer containing HRP-conjugated secondary antibody (1:2,000) at room temperature for 1 hour. After LumiGLO incubation, the blot was exposed to X-ray film for photographing.
Statistics
Data were expressed as mean ± SD, and statistical analysis was performed using ANOVA. A P value of less than 0.05 was considered significant.
Results
The effects of fluid shear stress on IL-8 gene expression in human ovarian cancer cells
When SKOV3 cells were exposed to low shear stresses (0.5, 1.5, or 2.0 dyne/cm2), the baseline values of IL-8 mRNA expression were 5.219×103, 5.308×103, and 5.038×103 copies, respectively. Throughout the monitoring course of the study, the expression increased when SKOV3 cells exposed to fluid shear stress for 1 h, reached the summits at 2 h, gradually decreased at 3 h and remained at a constant level at 4–12 h. The increase of IL-8 mRNA expression by shear stress was time-dependent. The biphasic response of IL-8 mRNA expression was found in the experiments in which the applied low shear stress was 0.5, 1.5, or 2.0 dyne cm2, respectively (Figure 1A).
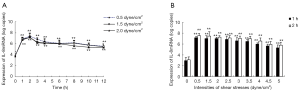
In order to assess the relationship between the intensity of the fluid shear stress and the expression of IL-8 mRNA, we exposed SKOV3 cells to different FSS (0.5, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, or 5.0 dyne/cm2 respectively), the results showed that IL-8 expression was negatively associated with the intensity of shear stress. After SKOV3 cells were exposed to low fluid shear stress (1.5 dyne/cm2) for 1 h and 2 h, IL-8 mRNA expression increased to near 68 and 52 times respectively as that of SKOV3 cells exposed to a high fluid shear stress of 5.0 dyne/cm2. The linear regression equations between IL-8 mRNA expression [log (copies), y] and shear stress (dyne/cm2, x) were: y=7.57−0.11x, r=−0.97 (for 1 h); y=7.92−0.10x, r=−0.96 (for 2 h) (Figure 1B).
The effects of fluid shear stress on IL-8 protein production in SKOV3 cells
SKOV3 cells untreated with fluid shear stress secreted very little IL-8 in culture media. IL-8 secretion increased obviously when fluid shear stress (0.5, 1.5, or 2.0 dyne/cm2) was exerted on SKOV3 cells for 1 h, which was 3.1, 3.3, 3.2-fold compared with that of static cells, respectively. The secretion reached the summit when SKOV3 cells exposed to fluid shear stress for 5 h, then IL-8 secretion gradually decreased at 8 h of stimulation by shear stress. IL-8 secretion remained at a constant level throughout the rest time course of the study. For all the groups tested in this study, it was found that the productions of IL-8 protein in SKOV3 cells subjected to shear stress are all time-dependent (Figure 2A).
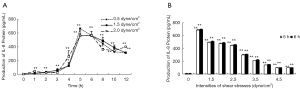
We had also shown the relationship between the intensity of the fluid shear stress and the protein production of IL-8. SKOV3 cells untreated with fluid shear stress secreted very little IL-8 in culture media. IL-8 secretion increased obviously when SKOV3 cells subjected to fluid shear stress (0.5, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0 dyne/cm2) for 5 h and still increased when exposed for 6 h. The secretion of IL-8 was the highest when SKOV3 cells subjected to fluid shear stress 1.5 dyne/cm2, which was near 6 or 7 times as that of SKOV3 cells subjected to high fluid shear stress (5.0 dyne/cm2). The linear regression equations between IL-8 protein production (y) and shear stress (dyne/cm2, x) were y=760.12−36.06x, r=−0.978 (for 5 h); y=781.87−36.66x, r=−0.980 (for 6 h) (Figure 2B).
Enhancement of green fluorescent protein expression by shear stress in the IL-8 reporter gene pEGFP1-IL8USCS-transfected SKOV3 cells
Flow cytometry analysis showed that when exposed to a shear stress of 1.5 dyne/cm2 for 3 hours, there was a significant increase in the enhanced green fluorescent protein expression in pEGFP1-IL8USCS-transfected endothelial cells (Figure 3), suggesting a flow shear stress-induced IL-8 gene transcriptional activation.

CXCR2 took part in the expression of IL-8 mRNA
To investigate whether CXCR2 presented on the surface of SKOV3 cells, we employed immunocytofluorescent staining, RT-PCR, Northern blot analysis. As shown in Figure 4A,B, CXCR2 immunocytofluorescent staining of SKOV3 cells showed that CXCR2 was present on the surface of SKOV3 cells (Figure 4A). Both RT-PCR detection and Northern blot analysis proved that SKOV3 cells constitutively expressed CXCR1 and CXCR2 mRNA. However, when exposing the cells to 1.5 dyne/cm2 flow shear stress for 1 hour, expression of CXCR2 mRNA was enhanced (Figure 4B), suggesting that the shear stress promotes the CXCR2 production on SKOV3 cells. Meanwhile, there was almost no change in CXCR1 gene expression. Furthermore, we found intracellular domain-truncated CXCR2 inhibited IL-8 reporter gene expression in SKOV3 cells. When exposed to 1.5 dyne/cm2 flow shear stress for 3 hours, Flow Cytometric analysis showed that there was almost no change in the level of enhanced green fluorescent protein expression in endothelial cells co-transfected with pcDNA3-mt CXCR2 and pEGFP1-IL8USCS (Figure 4C), indicating that the CXCR2 dominant-negative mutant inhibited the shear stress-induced IL-8 gene transcriptional activation.
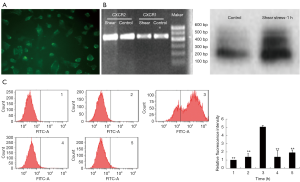
Shear stress-induced NF-κB activation in SKOV3 cells
Western blot of the cell lysates showed an increased density of P-IkB on the blot exposing to 1.5 dyne/cm2 flow shear stress for 10 minutes. However, it declined dramatically after 60 minutes of exposure. On the contrary, the density of IkB blot decreased with increasing exposure time (Figure 5A). These results showed that low shear stress induces IkB phosphorylation and degradation, resulting in NF-κB activation, on SKOV3 cells.
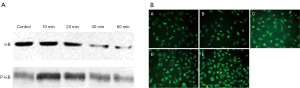
NF-κB p65 immunofluorescence of SKOV3 cells showed that in the untreated cells, there was cytoplasmic staining, but no nuclear staining. In contrast, when treated with 1.5 dyne/cm2 flow shear stress for 0.5 or 1 hour, the cell nuclei became staining; after 1.5 or 2 hours, the staining increased significantly, justifying that NF-κB immigrated from the cytoplasm into the nucleus (Figure 5B).
Discussion
Ovarian cancer cells are exposed to low fluid shear stress in the peritoneal cavity during both tumor growth and invasion, and the ascites in metastatic ovarian cancer further increases the exposure of ovarian cancer cells to fluid shear stress. It is of great significance to study the effect of fluid shear stress on ovarian cancer. Here, we use the estimated magnitude of ascites motion in the peritoneal cavity to investigate the changes caused by FSS in ovarian cancer cells. In fact, tumor mechanosensing involves a mechanical interplay between cancer cells, extracellular matrix, and cytokines or chemokines (including their receptors and mechano-induced transcriptional regulators) in the tumor microenvironment. When mechanoreceptors sense alterations of the extracellular mechanical cues, the signaling molecules are activated by cytokines or chemokines to mediate oncogenic transcription in favor of cancer initiation, survival, growth, and metastasis. From our study, we found that low flow shear stress-induced IL-8 gene transcriptional activation and protein production. Following the application of shear stress of 1.5 dyne/cm2, IL-8 mRNA expression and protein production increased with the peaks at 1 and 2 h respectively. Also, there was an increase in enhanced green fluorescent protein expression in pEGFP1-IL8USCS-transfected SKOV3 cells subjected to a fluid shear stress of 1.5 dyne/cm2 for 2 h. Therefore, IL-8 plays a key role in the low flow shear stress-induced biological processes in the ovarian cancer cell. In fact, in ovarian cancer, cytokines and chemokines with tumor development have been reported to exert autocrine effects to regulate the proliferation, angiogenesis, and metastasis (22). IL-8 as a proangiogenic cytokine was a molecular determinant of growth and progression in ovarian cancer, and its secretion may enhance a series of related behaviors of proliferation, angiogenesis, tumor adhesion, and invasion in ovarian cancer (23,24). IL-8 and its receptors were expressed in ovarian cancer, and there was a significant correlation between the expression and tumor stage and tumor type (25). Patients with malignant ovarian cancer had higher IL-8 levels compared to patients with benign neoplasms (26). In the evaluation of IL-8 and prognostic factors in ovarian cancer, increased levels of IL-8 were associated with factors of worse prognosis in ovarian cancer (27). Moreover, inhibition of IL-8 signaling may enhance the response to the anticancer drugs, such as platinum and Bortezomib (28,29). Thus, to our knowledge, IL-8 was related to the fluid shear stress-activated biological pathway and involved in the malignant biological behavior of ovarian cancer.
We also evaluate the effects of low fluid shear stress on the expression of IL-8 receptors. CXCR1 and CXCR2 are two G protein-coupled receptors normally located on the surface of leukocytes and endothelial cells, which can be activated by IL-8 or other chemokines, thus mediating multiple intracellular signaling pathways (30). CXCR1 and CXCR2 are also found on the surface of the ovarian cancer cells, and mounting evidence suggests that CXCR2 is the main receptor of IL-8 in ovarian cancer cells (31-33). Upregulation of CXCR2 has been shown to promote tumor progression in ovarian cancers, and the high expression of CXCR2 was significantly correlated with lower progression-free survival (PFS) and post-progression survival (PPS), indicating the importance of CXCR2 in affecting ovarian cancer recurrence and metastasis (32,34). To confirm CXCR2 presented on the surface of SKOV3 cells, we employed immunofluorescence and found CXCR2 was present on the surface of SKOV3 cells. CXCR2 was also considered to be the mechano-sensors of endothelial cells to sense fluid shear stress (21). To confirm the role of CXCR2 in sensing fluid shear stress in the ovarian cancer cell, we exposed the cells to 1.5 dyne/ cm2 flow shear stress. Notably, expression of CXCR2 mRNA was enhanced when exposed to flow shear stress, while there was almost no change in CXCR1 gene expression, suggesting that the flow shear stress promotes the CXCR2 production on SKOV3 cells. Furthermore, the CXCR2 dominant-negative mutant inhibited the shear stress-induced IL-8 gene transcriptional activation, suggesting the role of CXCR2 in FSS/IL-8-mediated ovarian cancer cell aggressiveness. Therefore, under induced by fluid shear stress, CXCR2 might sense the mechanical signal to promote malignancy of ovarian cancers via IL-8.
The signaling pathway, which induced IL-8 expression when exposed to fluid shear stress, was also be investigated. The IL-8 promoter region contains binding sites for the transcription factors NF-κB, and abundant research has demonstrated that NF-κB pathway is the essential pathway involved in IL-8 expression regulation in human tumors (35-37). Thus, we checked the involvement of the NF-κB pathway in FSS-induced IL-8 upregulation in ovarian cancer cells. To track NF-κB activation, we also checked the NF-κB P65 translocation. We found the low shear stress-induced IkB phosphorylation and degradation, and induced NF-κB P65 translocation from the cytoplasm to the nucleus, resulting in NF-kB activation. Thus, our results proved that the response of IL-8 in SKOV3 cells to fluid shear stress represented an early gene activation, and the activation can be mediated through NF-κB.
Conclusions
Collectively, the evidence suggested a relationship between increased levels of IL-8 and low fluid shear stress in ovarian cancer. And the results also confirmed that CXCR2 and NF-κB were the key signaling components in mechanotransduction pathways of the fluid shear stress-induced expression of IL-8. Given that low fluid shear stress, CXCR2 and NF-κB could effectively mediate IL-8 expression in ovarian cancer, and it was of significant importance to target low fluid shear stress, CXCR2 or NF-κB to control the expression of IL-8. There have been reports demonstrating that diminishing NF-κB or blocking the CXCR2 could inhibit inflammatory responses and cancer cell proliferation (34,38). And prolonged high shear stress treatment could effectively reduce the viability of highly metastatic and drug-resistant breast cancer cells (39). Thus, our studies provide mechanosensitive molecules as potential therapeutic targets for ovarian cancer. Further clarifying the role of these mechanosensitive components in the tumor microenvironment will offer a unique approach to target inflammation and metastasis of ovarian carcinomas.
Acknowledgments
Funding:
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. [Crossref] [PubMed]
- Liao J, Qian F, Tchabo N, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One 2014;9:e84941. [Crossref] [PubMed]
- Hou MM, Chen Y, Wu YK, et al. Pathological characteristics and prognosis of 664 patients with epithelial ovarian cancer: a retrospective analysis. Sichuan Da Xue Xue Bao Yi Xue Ban 2014;45:859-62, 875. [PubMed]
- Shield K, Ackland ML, Ahmed N, et al. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol Oncol 2009;113:143-8. [Crossref] [PubMed]
- Cohen CA, Shea AA, Heffron CL, et al. The parity-associated microenvironmental niche in the omental fat band is refractory to ovarian cancer metastasis. Cancer Prev Res (Phila) 2013;6:1182-93. [Crossref] [PubMed]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053-64. [Crossref] [PubMed]
- Avraham-Chakim L, Elad D, Zaretsky U, et al. Fluid-flow induced wall shear stress and epithelial ovarian cancer peritoneal spreading. PLoS One 2013;8:e60965. [Crossref] [PubMed]
- Heng HH, Stevens JB, Bremer SW, et al. Evolutionary mechanisms and diversity in cancer. Adv Cancer Res 2011;112:217-53. [Crossref] [PubMed]
- Chotard-Ghodsnia R, Haddad O, Leyrat A, et al. Morphological analysis of tumor cell/endothelial cell interactions under shear flow. J Biomech 2007;40:335-44. [Crossref] [PubMed]
- Dong C, Slattery M, Liang S. Micromechanics of tumor cell adhesion and migration under dynamic flow conditions. Front Biosci 2005;10:379-84. [Crossref] [PubMed]
- Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol 2005;288:C831-9. [Crossref] [PubMed]
- Rizvi I, Gurkan UA, Tasoglu S, et al. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci U S A 2013;110:E1974-83. [Crossref] [PubMed]
- Jeffrey B, Udaykumar HS, Schulze KS. Flow fields generated by peristaltic reflex in isolated guinea pig ileum: impact of contraction depth and shoulders. Am J Physiol Gastrointest Liver Physiol 2003;285:G907-18. [Crossref] [PubMed]
- Ruan Y, Ji X, Wen M, et al. Interleukin 8 enhances the immune response of ducks to avian influenza vaccine. Acta Virol 2014;58:356-8. [Crossref] [PubMed]
- Alfaro C, Sanmamed MF, Rodriguez-Ruiz ME, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev 2017;60:24-31. [Crossref] [PubMed]
- Kosmopoulos M, Christofides A, Drekolias D, et al. Critical Role of IL-8 Targeting in Gliomas. Curr Med Chem 2018;25:1954-67. [Crossref] [PubMed]
- Lee KE, Khoi PN, Xia Y, et al. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol 2013;19:8192-202. [Crossref] [PubMed]
- Shathasivam P, Kollara A, Spybey T, et al. VEPH1 expression decreases vascularisation in ovarian cancer xenografts and inhibits VEGFA and IL8 expression through inhibition of AKT activation. Br J Cancer 2017;116:1065-76. [Crossref] [PubMed]
- Ishibazawa A, Nagaoka T, Yokota H, et al. Low shear stress up-regulation of proinflammatory gene expression in human retinal microvascular endothelial cells. Exp Eye Res 2013;116:308-11. [Crossref] [PubMed]
- Ye D, Liang W, Dai L, et al. Moderate Fluid Shear Stress Could Regulate the Cytoskeleton of Nucleus Pulposus and Surrounding Inflammatory Mediators by Activating the FAK-MEK5-ERK5-cFos-AP1 Signaling Pathway. Dis Markers 2018;2018:9405738. [PubMed]
- Zeng Y, Sun HR, Yu C, et al. CXCR1 and CXCR2 are novel mechano-sensors mediating laminar shear stress-induced endothelial cell migration. Cytokine 2011;53:42-51. [Crossref] [PubMed]
- Barbieri F, Bajetto A, Florio T. Role of chemokine network in the development and progression of ovarian cancer: a potential novel pharmacological target. J Oncol 2010;2010:426956. [Crossref] [PubMed]
- Wang SC, Fu HH, Wen JR, et al. Sichuan Da Xue Xue Bao Yi Xue Ban 2018;49:420-4. [IL-8 Induces Epithelial-to-mesenchymal Transition of Ovarian Carcinoma Cells: a Preliminary Study]. [PubMed]
- Wang Y, Xu RC, Zhang XL, et al. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine 2012;59:145-55. [Crossref] [PubMed]
- Browne A, Sriraksa R, Guney T, et al. Differential expression of IL-8 and IL-8 receptors in benign, borderline and malignant ovarian epithelial tumours. Cytokine 2013;64:413-21. [Crossref] [PubMed]
- Martins-Filho A, Jammal MP, Micheli DC, et al. Role of Intracystic Cytokines and Nitric Oxide in Ovarian Neoplasms. Scand J Immunol 2017;86:462-70. [Crossref] [PubMed]
- Sanguinete MMM, Oliveira PH, Martins-Filho A, et al. Serum IL-6 and IL-8 Correlate with Prognostic Factors in Ovarian Cancer. Immunol Invest 2017;46:677-88. [Crossref] [PubMed]
- Singha B, Gatla HR, Phyo S, et al. IKK inhibition increases bortezomib effectiveness in ovarian cancer. Oncotarget 2015;6:26347-58. [Crossref] [PubMed]
- Stronach EA, Cunnea P, Turner C, et al. The role of interleukin-8 (IL-8) and IL-8 receptors in platinum response in high grade serous ovarian carcinoma. Oncotarget 2015;6:31593-603. [Crossref] [PubMed]
- Hidaka H, Ishiko T, Furuhashi T, et al. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface:impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer 2002;95:1206-14. [Crossref] [PubMed]
- Agarwal A, Tressel SL, Kaimal R, et al. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Res 2010;70:5880-90. [Crossref] [PubMed]
- Yang G, Rosen DG, Liu G, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res 2010;16:3875-86. [Crossref] [PubMed]
- Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate 1999;41:78-88. [Crossref] [PubMed]
- Yung MM, Tang HW, Cai PC, et al. GRO-alpha and IL-8 enhance ovarian cancer metastatic potential via the CXCR2-mediated TAK1/NFkappaB signaling cascade. Theranostics 2018;8:1270-85. [Crossref] [PubMed]
- Elliott CL, Allport VC, Loudon JA, et al. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod 2001;7:787-90. [Crossref] [PubMed]
- Park JY, Chang JH, Bae KS, et al. NF-kappaB-dependency and consequent regulation of IL-8 in echinomycin-induced apoptosis of HT-29 colon cancer cells. Cell Biol Int 2008;32:1207-14. [Crossref] [PubMed]
- Wang S, Liu Z, Wang L, et al. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol 2009;6:327-34. [Crossref] [PubMed]
- Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci U S A 2013;110:972-7. [Crossref] [PubMed]
- Regmi S, Fu A, Luo KQ. High Shear Stresses under Exercise Condition Destroy Circulating Tumor Cells in a Microfluidic System. Sci Rep 2017;7:39975. [Crossref] [PubMed]


