Neoadjuvant chemotherapy-induced severe neutropenia is associated with histopathological response and survival in locally advanced gastric cancer
Introduction
Neoadjuvant chemotherapy (NAC) significantly improves the resectability and survival outcomes of patients with potentially resectable locally advanced gastric cancer (LAGC) through tumor regression and tumor downstaging (1,2). Histopathological response (HPR), a surrogate for chemotherapy efficacy, is a promising prognostic factor for patients treated with NAC combined with surgery (3). Based on the ratio of fibrosis to residual tumor after NAC, one of the most common measures of HPR is the tumor regression grade (TRG). Favorable HPR, reported in only 33–57% of patients, has been found to serve as an indicator for better clinical outcomes (4-6). Moreover, evaluation of tumor regression after NAC may be beneficial for decision-making regarding postoperative chemotherapy regimens (7,8). Nonetheless, NAC frequently impairs both nutritional status and physical fitness, which may predispose patients towards an elevated risk of postoperative morbidity and mortality (9,10). Therefore, distinguishing responders from non-responders as early as possible will help clinicians prevent unnecessary chemotherapy and adopt more effective regimens or surgical resection.
Neutropenia is the most common chemotherapy-related adverse event and correlates with favorable tumor responses and/or better survival in neoadjuvant, adjuvant, and palliative settings for several tumor types, such as colorectal cancer and esophageal cancer (11-14). These findings indicate that neutropenia, a reflection of the host response to the administration of chemotherapy, may be closely related to tumor response or prognosis. However, data regarding the impact of neutropenia on tumor response and prognosis in LAGC patients treated with NAC are quite limited. In this study, we aimed to investigate the relationship between NAC-induced neutropenia and clinicopathological variables and examine the impact of NAC-induced neutropenia on therapeutic outcomes.
Methods
Patients and treatments
This was a monocentric study that retrospectively collected data from 233 patients treated with NAC followed by surgery for primary LAGC between 2006 and 2016. All patients had pathologically confirmed gastric adenocarcinoma, and patients with any other active synchronous tumors excluded. The Institutional Review Board of National Cancer Centre/Cancer Hospital reviewed and approved this study and agreed that individual patient consent was not required to report clinical outcomes alone.
The preoperative chemotherapy regimens at our centre included S-1 plus oxaliplatin (SOX) or capecitabine plus oxaliplatin (XELOX). For patients tolerate it well, paclitaxel was added to the SOX or XELOX regimen according to the oncologists’ decision. Dosage reduction, treatment postponement or interruption was considered in cases of severe adverse events. If patients did not respond to preoperative chemotherapy, switching to other regimens or surgical resection was considered after informed consent was obtained. Total or subtotal gastrectomy plus D2-lymph node dissection was performed according to the guidelines of the Japanese Gastric Cancer Association. Additional organ resection was performed in cases of adjacent organ involvement. Adjuvant chemotherapy was initiated 4–6 weeks after the surgery, and the regimen was the same as that of NAC. Adjuvant chemotherapy was postponed or cancelled in cases of severe chemotherapy toxicity, postoperative complications, impaired nutrition status, or other reasons.
Assessments
Before surgery, the anti-tumor effect was assessed every two cycles according to Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1). A clinical response was defined as either complete response (CR) or partial response (PR); a non-response was defined as either stable disease (SD) or progressive disease (PD) (15). Chemotherapy-related neutropenia within 3 weeks of every cycle of chemotherapy was graded by clinicians according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (16). If an adverse event occurred with multiple grades across various cycles, only the worst grade was registered. Grade 1 neutropenia was equal to a neutrophil count between the lower limit of normal and 1,500 cells/mL, grade 2 between 1,500 and 1,000 cells/mL, grade 3 between 1,000 and 500 cells/mL, and grade 4 less than 500 cells/mL. Grade 3/4 neutropenia was defined as severe, and grade 1/2 neutropenia was defined as mild. Administration of granulocyte colony-stimulating factor (G-CSF) was considered for severe neutropenia in accordance with established guidelines, and prophylactic administration was not allowed (17,18). Each postoperative complication was allocated a severity grade using the Clavien-Dindo classification system. If multiple morbidities occurred in one patient, the highest grade was used.
Regarding pathological response, each tumor was allocated a TRG score as described by Mandard: 1, an absence of residual cancer and a large amount of fibrosis; 2, a few residual cancer cells scattered throughout the fibrosis; 3, more residual tumor cells but fibrosis predominated; 4, residual cancer cells predominated over fibrosis; and 5, no signs of regression (19). Favorable HPR was defined as a TRG score of 1–3; unfavorable HPR was defined as a TRG score of 4–5.
Follow-up
The anti-tumor effect was evaluated for every patient every two cycles prior to surgery. After surgery, patients were followed up every 3 months during the first 2 postoperative years, every 6 months thereafter for 3 years, and yearly after 5 years. Recurrence and death were determined from hospital records or from telephone interviews. Disease-free survival (DFS) was calculated as the time interval between the date of surgery and confirmation of the first recurrence by imaging or pathological diagnosis. Overall survival (OS) was calculated as the time interval from surgery to the time of death for any reason.
Statistical analysis
Categorical variables were analysed using the chi-square or Fisher’s exact test, and continuous data were analysed using Student’s t-test or Mann-Whitney U test. Survival was assessed by Kaplan-Meier estimates and compared using the log-rank test. The association between clinicopathological factors and outcome (i.e., responders vs. non-responders) was explored using binary logistic regression analysis. Cox regression models were applied to explore the association between NAC-related severe neutropenia and survival outcomes after adjustment for potential confounders. Covariates with P<0.1 in univariate analysis were examined in multivariable analysis (backward selection strategy using a likelihood ratio statistic). All statistical tests were conducted using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at 2-sided P<0.05.
Results
Patient and tumor characteristics
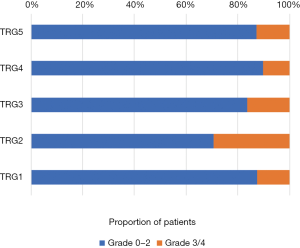
The characteristics of the 233 patients who participated in this study are shown in Table 1. NAC-induced neutropenia was observed in 43.8% (102/233) of the patients and NAC-induced severe neutropenia (NISN) in 15.0% (35/233). The median number of cycles of NAC was 4 [interquartile range (IQR), 3–4]. According to RECIST criteria, 165 (70.8%) patients showed PR, and 68 (29.2%) patients showed SD. No patients showed CR or PD. The median number of cycles of postoperative chemotherapy among patients treated with adjuvant chemotherapy was 4 (IQR 2–6). TRG results for patients with NISN were as follows: TRG 1 (n=2, 5.7%); TRG 2 (n=10, 28.6%); TRG 3 (n=8, 22.8%); TRG 4 (n=9, 25.7%); and TRG 5 (n=6, 17.1%). The results for patients without NISN were as follows: TRG 1 (n =14, 7.1%); TRG 2 (n=24, 12.1%); TRG 3 (n=41, 20.7%); TRG 4 (n=78, 39.4%); and TRG 5 (n=41, 20.7%) (Figure S1).
Table 1
| Variables | All (n=233) | Grade 0–2 neutropenia (n=198) | Grade 3/4 neutropenia (n=35) | P value |
|---|---|---|---|---|
| Gender, n (%) | 0.728 | |||
| Male | 159 (68.2) | 136 (68.7) | 23 (65.7) | |
| Female | 74 (31.8) | 62 (31.3) | 12 (34.3) | |
| Age, n (%) | 0.742 | |||
| <65 years | 191 (82.0) | 163 (82.3) | 28 (80.0) | |
| ≥65 years | 42 (18.0) | 35 (17.7) | 7 (20.0) | |
| ASA risk score, n (%) | 0.848 | |||
| 1–2 | 211 (90.6) | 179 (90.4) | 32 (91.4) | |
| 3–4 | 22 (9.4) | 19 (9.6) | 3 (8.6) | |
| cT, n (%) | 0.175 | |||
| T1–2 | 10 (4.3) | 7 (3.5) | 3 (8.6) | |
| T3–4 | 223 (95.7) | 191 (96.5) | 32 (91.4) | |
| cN, n (%) | 0.598 | |||
| N0 | 26 (11.2) | 23 (11.6) | 3 (8.6) | |
| N+ | 207 (88.8) | 175 (88.4) | 32 (91.4) | |
| Regimen of NAC, n (%) | 0.100 | |||
| Double | 122 (52.4) | 109 (55.1) | 14 (40.0) | |
| Triple | 111 (47.6) | 89 (44.9) | 21 (60.0) | |
| No. of NAC cycles, n (%) | 0.241 | |||
| <4 cycles | 101 (43.3) | 89 (45.0) | 14 (34.3) | |
| ≥4 cycles | 132 (56.7) | 109 (55.0) | 23 (65.7) | |
| Clinical response, n (%) | 0.471 | |||
| Response | 165 (70.8) | 142 (71.7) | 23 (65.7) | |
| Non-response | 68 (29.2) | 56 (28.3) | 12 (34.3) | |
| Approach, n (%) | 0.040* | |||
| Open | 191 (82.0) | 158 (79.8) | 33 (94.3) | |
| Laparoscopic | 42 (18.0) | 40 (20.2) | 2 (5.7) | |
| Extent of gastrectomy, n (%) | 0.136 | |||
| Subtotal | 146 (62.7) | 128 (64.6) | 18 (51.4) | |
| Total | 87 (37.3) | 70 (35.4) | 17 (48.6) | |
| Additional organs resection, n (%) | 13 (5.58) | 12 (6.1) | 1 (2.9) | 0.447 |
| Tumor location, n (%) | 0.617 | |||
| Upper | 58 (24.9) | 49 (24.7) | 9 (25.7) | |
| Middle | 69 (29.6) | 61 (30.8) | 8 (22.9) | |
| Low | 106 (45.5) | 88 (44.4) | 18 (51.4) | |
| Differentiation, n (%) | 0.546 | |||
| Well/moderate | 63 (27.0) | 55 (27.8) | 8 (22.9) | |
| Poor/undifferentiated | 170 (73.0) | 143 (72.2) | 27 (77.1) | |
| LVI, n (%) | 58 (24.9) | 51 (25.8) | 7 (20.0) | 0.468 |
| HPR, n (%) | 0.002* | |||
| 1–3 | 103 (44.2) | 79 (39.9) | 24 (68.6) | |
| 4–5 | 130 (55.8) | 119 (60.1) | 11 (31.4) | |
| pT, n (%) | 0.837 | |||
| T0–2 | 70 (30.0) | 60 (30.3) | 10 (28.6) | |
| T3–4 | 163 (70.0) | 138 (69.7) | 25 (71.4) | |
| pN, n (%) | 0.312 | |||
| N0 | 76 (32.6) | 62 (31.3) | 14 (40.0) | |
| N+ | 157 (67.4) | 136 (68.7) | 21 (60.0) | |
| No. of dissected LNs, median [IQR] | 29 [21–38.5] | 29 [21–39] | 29 [20–37] | 0.686 |
| No. of metastatic LNs, median [IQR] | 2 [0–6.5] | 2 [0–7] | 2 [0–6] | 0.552 |
| Residual tumor (R0), n (%) | 218 (93.6) | 186 (93.9) | 32 (91.4) | 0.577 |
| R+, n (%) | 15 (6.3) | 12 (6.1) | 3 (8.6) | |
| Postop complications, n (%) | 0.239 | |||
| None | 166 (71.2) | 139 (70.2) | 27 (77.1) | |
| I–II | 52 (22.3) | 44 (22.2) | 8 (22.9) | |
| III–IV | 15 (6.4) | 15 (7.6) | 0 (0) | |
| Postop chemotherapy (yes), n (%) | 171 (73.4) | 146 (73.7) | 25 (71.4) | 0.776 |
*, results are shown statistically significant. NISN, neoadjuvant chemotherapy-induced severe neutropenia; ASA, American Society of Anesthesiologists; NAC, neoadjuvant chemotherapy; LVI, lymphovascular invasion; postop, postoperative.
Relationship between HPR and clinicopathological features
Relationships between HPR and clinicopathological features were analysed, and the results are shown in Table 2. Univariate analysis revealed that the NAC regimen, tumor differentiation, lymphovascular invasion (LVI), pathological (p) T, pN, clinical response, and grade of neutropenia correlated with HPR. Multivariate analysis identified well/moderate differentiation [odds ratio (OR), 2.811, 95% confidence interval (CI): 1.444–5.470, P=0.002], clinical response (OR 2.342, 95% CI: 1.193–4.598, P=0.013), absence of LVI (OR 3.597, 95% CI: 1.724–7.519, P=0.001) and NISN (OR 4.158, 95% CI: 1.762–9.812, P=0.001) as independent predictors of a favorable HPR (Table 2).
Table 2
| Variables | Unfavorable HPR (n=130) | Favorable HPR (n=103) | P value |
|---|---|---|---|
| Univariate analysis, n (%) | |||
| Gender | 0.182 | ||
| Male | 84 (64.6) | 75 (72.8) | |
| Female | 46 (35.4) | 28 (27.2) | |
| Age | 0.623 | ||
| <65 years | 108 (83.1) | 83 (80.6) | |
| ≥65 years | 22 (16.9) | 20 (19.4) | |
| ASA risk score | 0.219 | ||
| 1–2 | 115 (88.5) | 96 (93.2) | |
| 3–4 | 15 (11.5) | 7 (6.8) | |
| cT | 0.784 | ||
| T1–2 | 6 (4.6) | 4 (3.9) | |
| T3–4 | 124 (95.4) | 99 (96.1) | |
| cN | 0.143 | ||
| N0 | 18 (13.8) | 8 (7.8) | |
| N+ | 112 (86.2) | 95 (92.2) | |
| Regimen of NAC | 0.067 | ||
| Double | 75 (57.7) | 47 (45.6) | |
| Triple | 55 (42.3) | 56 (54.1) | |
| No. of NAC cycles | 0.925 | ||
| <4 cycles | 56 (43.1) | 45 (43.7) | |
| ≥4 cycles | 74 (56.9) | 58 (56.3) | |
| Clinical response | 0.001* | ||
| Response | 81 (62.3) | 84 (81.6) | |
| Non-response | 49 (37.7) | 19 (18.4) | |
| Differentiation | <0.001* | ||
| Well/moderate | 22 (16.9) | 41 (39.8) | |
| Poor/undifferentiated | 108 (83.1) | 62 (60.2) | |
| Tumor location | 0.761 | ||
| Upper | 31 (23.8) | 27 (26.2) | |
| Middle | 41 (31.5) | 28 (27.2) | |
| Low | 58 (44.6) | 48 (46.6) | |
| pT | <0.001* | ||
| T0–2 | 21 (16.2) | 49 (47.6) | |
| T3–4 | 109 (83.8) | 54 (52.4) | |
| pN | <0.001* | ||
| N0 | 28 (21.5) | 48 (46.6) | |
| N+ | 102 (78.5) | 55 (53.4) | |
| LVI | 46 (35.4) | 12 (11.7) | <0.001* |
| NISN | 11 (8.5) | 24 (23.3) | 0.002* |
| Multivariate analysis# | |||
| Differentiation (well/moderate) | 2.811 | 1.444–5.470 | 0.002 |
| Clinical response (response) | 2.342 | 1.193–4.598 | 0.013 |
| LVI (absent) | 3.597 | 1.724–7.519 | 0.001 |
| NISN | 4.158 | 1.762–9.812 | 0.001 |
Data below “Multivariate analysis” are presented as OR, 95% CI, P value. *, results are shown statistically significant; #, pT, and pN were not included as these variables could not be confirmed prior to surgery. HPR, histopathological response; OR, odds ratio; CI, confidence interval; IQR, interquartile range; NISN, neoadjuvant chemotherapy-induced severe neutropenia.
Survival outcomes
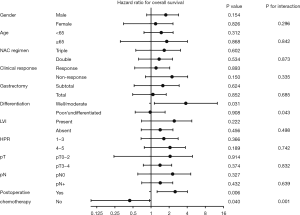
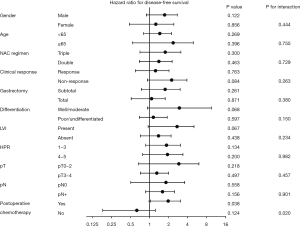
The median follow-up time for the 233 patients was 46.3 (95% CI: 40.1–52.4) months. During the follow-up period, 118 patients (50.6%) developed recurrence, and 99 patients (42.5%) died. The median DFS and OS for the entire cohort were 32.1 (95% CI: 19.6–44.6) and 56.8 (95% CI: 35.8–77.7) months, respectively. NISN did not affect OS [hazard ratio (HR) 1.278, 95% CI: 0.733–2.220, P=0.345) or DFS (HR 1.266, 95% CI: 0.759–2.110, P=0.325) in the entire cohort (Figure 1). The median DFS was 66.2 (95% CI: 33.4–98.9) months in patients with a favorable HPR and 23.3 (95% CI: 14.8–31.9) months in those with an unfavorable HPR (P=0.019). The median OS was not reached in those with a favorable HPR, and was 44.6 (95% CI: 21.9–67.2) months in those with an unfavorable HPR (P=0.036).
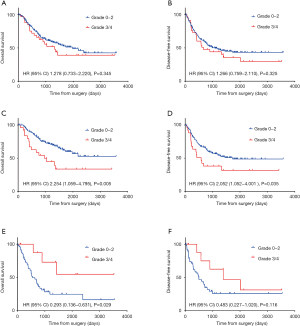
Subgroup analysis of survival revealed a significant interaction between NISN and postoperative chemotherapy (Figures 2,S2). NISN correlated with poor OS (HR 2.254, 95% CI: 1.059–4.795, P=0.005) and poor DFS (HR 2.052, 95% CI: 1.052–4.001, P=0.035) in patients treated with postoperative chemotherapy (Figure 1C,D). The 3-year OS and DFS rates were 44.9% and 38.1% for patients with NISN and 71.6% and 56.5% for patients without NISN, respectively. However, among patients treated with preoperative chemotherapy alone, NISN was associated with a better OS (HR 0.293, 95% CI: 0.136–0.631, P=0.029) and a tendency towards a better DFS (HR 0.483, 95% CI: 0.227–1.020, P=0.116) (Figure 1E,F). The 3-year OS and DFS rates were 72.9% and 62.5% for patients with NISN and 28.4% and 26.0% for patients without NISN, respectively.
NISN negatively affects compliance with postoperative chemotherapy
We further compared the clinicopathological characteristics of patients with NISN to those of patients without NISN. As illustrated in Table 3, NISN was associated with a higher proportion of open surgery (P=0.024), favorable HPR (P=0.005), and fewer cycles of postoperative chemotherapy (P=0.013). Table 4 suggests that open surgery (OR 0.467, 95% CI: 0.232–0.941, P=0.033) and NISN (OR 0.364, 95% CI: 0.148–0.894, P=0.028) were independently associated with poor compliance with postoperative chemotherapy (<4 cycles).
Table 3
| Variables | Grade 0-2 neutropenia (n=146) | Grade 3/4 neutropenia (n=25) | P value |
|---|---|---|---|
| Gender, n (%) | 0.300 | ||
| Male | 102 (69.8) | 20 (80.0) | |
| Female | 44 (30.1) | 5 (20.0) | |
*, results are shown statistically significant; #, details about No. of postop cycles were missing at nine patients.
Table 4
| Variables | <4 postop cycles (n=77) | ≥4 postop cycles (n=85) | P value |
|---|---|---|---|
| Univariate analysis, n (%) | |||
| Gender | 0.949 | ||
| Male | 54 (70.1) | 60 (70.6) | |
| Female | 23 (29.9) | 25 (29.4) | |
| Age | 0.363 | ||
| <65 years | 61 (79.2) | 72 (84.7) | |
| ≥65 years | 16 (20.8) | 13 (15.3) | |
| ASA risk score | 0.858 | ||
| 1–2 | 68 (88.3) | 76 (89.4) | |
| 3–4 | 9 (11.7) | 9 (10.6) | |
| cT | 0.858 | ||
| T1–2 | 6 (2.6) | 6 (5.9) | |
| T3–4 | 71 (97.3) | 79 (94.1) | |
| cN | 0.988 | ||
| N0 | 9 (11.7) | 10 (11.8) | |
| N+ | 68 (88.3) | 75 (88.2) | |
| Regimen of NAC | 0.054 | ||
| Double | 47 (61.0) | 39 (45.9) | |
| Triple | 30 (39.0) | 46 (54.1) | |
| No. of NAC cycles | 0.956 | ||
| <4 cycles | 35 (45.5) | 39 (54.1) | |
| ≥4 cycles | 42 (54.5) | 46 (45.9) | |
| Clinical response | 0.272 | ||
| Response | 61 (82.4) | 61 (71.8) | |
| Non-response | 16 (17.6) | 24 (28.2) | |
| NISN | 17 (22.1) | 7 (9.2) | 0.013* |
| Approach | 0.076 | ||
| Open | 65 (84.4) | 62 (72.9) | |
| Laparoscopic | 12 (15.6) | 23 (27.1) | |
| Extent of gastrectomy | 0.334 | ||
| Subtotal | 46 (59.7) | 57 (67.1) | |
| Total | 31 (40.3) | 28 (32.9) | |
| Additional organs resection | 6 (7.8) | 5 (5.9) | 0.629 |
| Tumor location | 0.510 | ||
| Upper | 20 (26.0) | 18 (21.2) | |
| Middle | 25 (32.5) | 24 (28.2) | |
| Low | 32 (41.6) | 43 (50.6) | |
| Differentiation | 0.106 | ||
| Well/moderate | 27 (35.1) | 20 (23.5) | |
| Poor/undifferentiated | 50 (64.9) | 65 (76.5) | |
| LVI | 16 (20.8) | 21 (24.7) | 0.552 |
| HPR | 0.918 | ||
| 1–3 | 45 (58.4) | 49 (57.6) | |
| 4–5 | 32 (41.6) | 36 (42.4) | |
| pT | 0.674 | ||
| T0–2 | 23 (29.9) | 28 (32.9) | |
| T3–4 | 54 (70.1) | 57 (67.1) | |
| pN | 0.824 | ||
| N0 | 25 (32.5) | 29 (34.1) | |
| N+ | 52 (67.5) | 56 (65.9) | |
| Residual tumor | 0.886 | ||
| R0 | 73 (94.8) | 81 (95.3) | |
| R+ | 4 (5.2) | 4 (4.7) | |
| Postop complications | 0.795 | ||
| None | 58 (75.3) | 60 (70.6) | |
| I–II | 16 (20.8) | 21 (24.7) | |
| III–IV | 3 (3.9) | 4 (4.7) | |
| Postop hospital stay, median [IQR] | 12 [9–14] | 11 [9–13] | 0.247 |
| Time to adjuvant chemotherapy, median [IQR] | 38 [33–44] | 33 [33–47] | 0.995 |
| Multivariate analysis | |||
| Approach (open) | 0.467 | 0.232−0.941 | 0.033* |
| NISN | 0.364 | 0.148−0.894 | 0.028* |
Data below “Multivariate analysis” are presented as OR, 95% CI, P value. *, results are shown statistically significant. NISN, neoadjuvant chemotherapy-induced severe neutropenia; NAC, neoadjuvant chemotherapy; IQR, interquartile range.
Impacts of NISN on survival
The results of univariate analysis regarding the OS and DFS are shown in Table 5. According to multivariate analysis (Table 6), the extent of gastrectomy (total gastrectomy, HR 2.545, 95% CI: 1.483–4.366, P=0.001), tumor differentiation (well/moderate, HR 0.417, 95% CI: 0.201–0.866, P=0.019), and pT (T3–4, HR 2.610, 95% CI: 1.198–5.689, P=0.016) were independently associated with OS among patients treated with postoperative chemotherapy. Tumor location (middle, HR 0.251, 95% CI: 0.134–0.471, P<0.001; lower, HR 0.254, 95% CI: 0.140–0.461, P<0.001), tumor differentiation (well/moderate, HR 0.203, 95% CI: 0.102–0.402, P<0.001), pT (T3–4, HR 1.974, 95% CI: 1.045–3.729, P=0.036), and pN (N+, HR 2.240, 95% CI: 1.221–4.111, P=0.009) were independently associated with DFS. The number of cycles of postoperative chemotherapy was an independent predictor of OS (≥4 cycles, HR 0.509, 95% CI: 0.297–0.871, P=0.014) and DFS (≥4 cycles, HR 0.609, 95% CI: 0.384–0.966, P=0.035), instead of NISN.
Table 5
| Variables | Both pre and postoperative chemotherapy | Preoperative chemotherapy only | |||
|---|---|---|---|---|---|
| P value for OS | P value for DFS | P value for OS | P value for DFS | ||
| Age | 0.378 | 0.526 | 0.133 | 0.107 | |
| Gender | 0.154 | 0.546 | 0.479 | 0.855 | |
| ASA risk score | 0.209 | 0.115 | 0.116 | 0.014* | |
| cT | 0.913 | 0.316 | 0.180 | 0.031* | |
| cN | 0.448 | 0.239 | 0.281 | 0.479 | |
| NAC regimens | 0.441 | 0.378 | 0.620 | 0.649 | |
| No. of NAC cycles | 0.462 | 0.233 | 0.074 | 0.391 | |
| Clinical response | 0.284 | 0.206 | 0.955 | 0.801 | |
| NISN | 0.005* | 0.035* | 0.029* | 0.116 | |
| Approach | 0.066 | 0.061 | 0.426 | 0.734 | |
| Extent of gastrectomy | <0.001* | <0.001* | 0.004* | 0.019* | |
| Additional organs resection | 0.594 | 0.436 | 0.366 | 0.243 | |
| Tumor location | 0.043* | 0.014* | 0.338 | 0.361 | |
| Differentiation | 0.002* | <0.001* | 0.018* | 0.009* | |
| LVI | 0.018* | 0.007* | 0.087 | 0.052 | |
| Residual tumor | 0.109 | 0.023* | 0.135 | 0.002* | |
| Postop complications | 0.347 | 0.019* | 0.057 | 0.241 | |
| HPR | 0.310 | 0.085 | 0.052 | 0.107 | |
| pT category | <0.001* | <0.001* | 0.016* | 0.023* | |
| pN category | 0.019* | 0.002* | 0.007* | 0.479 | |
| No. of postop cycles | 0.020* | 0.092 | NA | NA | |
*, results are shown statistically significant. OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; ASA, American society of Anesthesiologists; NAC, neoadjuvant chemotherapy; LVI, lymphovascular invasion; HPR, histopathological response; postop, postoperative. NA, not available.
Table 6
| Variables | Adjusted HR | 95% CI | P value |
|---|---|---|---|
| Both pre and postoperative chemotherapy | |||
| OS | |||
| Extent of gastrectomy (total) | 2.545 | 1.483–4.366 | 0.001* |
| Differentiation (well/moderate) | 0.417 | 0.201–0.866 | 0.019* |
| pT category (T3–4) | 2.610 | 1.198–5.689 | 0.016* |
| No. of postop cycles (≥4 cycles) | 0.509 | 0.297–0.871 | 0.014* |
| DFS | |||
| Tumor location (reference, upper) | |||
| Middle | 0.251 | 0.134–0.471 | <0.001* |
| Lower | 0.254 | 0.140–0.461 | <0.001* |
| Differentiation (well/moderate) | 0.203 | 0.102–0.402 | <0.001* |
| pT category (T3–4) | 1.974 | 1.045–3.729 | 0.036* |
| pN category (N+) | 2.240 | 1.221–4.111 | 0.009* |
| No. of postop cycles (≥4 cycles) | 0.609 | 0.384–0.966 | 0.035* |
| Preoperative chemotherapy only | |||
| OS# | |||
| NISN | 0.253 | 0.077–0.830 | 0.023* |
| Differentiation (well/moderate) | 0.195 | 0.046–0.824 | 0.026* |
| Extent of gastrectomy (total) | 2.309 | 1.181–4.516 | 0.014* |
*, results are shown statistically significant; #, multivariate analysis of DFS was not conducted as NISN was not a significant predictor in univariate analysis. OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; NAC, neoadjuvant chemotherapy; NISN, NAC-induced severe neutropenia; postop, postoperative.
Among patients treated with preoperative chemotherapy alone, NISN was an independent predictor of poor OS (HR 0.253, 95% CI: 0.077–0.830, P=0.023), in addition to the extent of gastrectomy (total gastrectomy, HR 2.309, 95% CI: 1.181–4.516, P=0.014) and tumor differentiation (well/moderate, HR 0.195, 95% CI: 0.046–0.824, P=0.026). The univariate analysis of DFS suggested that NISN was associated with a tendency towards a better survival (P=0.116).
Discussion
To our knowledge, this is the first study that attempts to investigate the effects of NISN on pathological response, treatment compliance and long-term survival in LAGC after NAC. Our findings demonstrate that NISN predicts a favorable HPR. Moreover, NISN confers a survival advantage on patients treated with preoperative chemotherapy alone. NISN also correlated with poor compliance to treatment and thus poor survival in patients treated with postoperative chemotherapy. These results might help to predict pathological response and improve prognostication, facilitating the selection of appropriate treatment strategies.
Published data have validated the ability of treatment-related neutropenia as a surrogate for treatment response and survival outcomes in neoadjuvant, adjuvant, and metastatic settings in many tumor types, such as colorectal cancer and esophageal cancer (11-14). This current study is the first to validate the potential of preoperative treatment-related neutropenia as a surrogate for a pathological response in LAGC treated with NAC followed by surgery. Severe neutropenia is suggestive of severe hematologic toxicity, and tumor regression refers to the degeneration of cancer tissues. The therapeutic effects of chemotherapeutic drugs usually occur in a dose-dependent but not tissue-specific manner. In other words, the hematologic system and cancerous tissues respond in a similar way to chemotherapy, which may be the reason for an association between neutropenia and pathological tumor regression. However, chemotherapy-induced neutropenia may reflect cytotoxic activity, representing delivery of an adequate dosage and thus an active anticancer effect. If severe neutropenia occurs, careful evaluation of clinical responses or biopsy-based HPR is necessary when deciding to continue preoperative chemotherapy with appropriate supportive treatments for neutropenia.
NISN independently predicted survival benefit among patients treated with preoperative chemotherapy alone, for which several mechanisms may be responsible. First, studies have suggested that neutrophils may be involved in the formation of a pre-metastatic microenvironment, facilitating progression, metastasis, colonization and treatment resistance by tumor cells (20-22). Consistent with the promotive role of neutrophils in tumor progression, treatment-related neutropenia has been correlated with superior survival (11-14). Second, many studies have found an association between histological tumor regression and better clinical outcomes, and our study corroborated their findings (23,24). When tumors respond to chemotherapy, cancer micrometastasis or occult metastasis that may not be eliminated by surgery can be effectively damaged. Moreover, we considered neutropenia as a measure of adequate chemotherapeutic dosing. Thus, the use of chemotherapy-induced neutropenia may ensure adequate dosing and benefit a large majority of patients who are currently receiving unintended chemotherapy underdosing.
Our findings suggest that NISN is independently associated with fewer cycles of postoperative chemotherapy and thus impairs survival among patients treated with postoperative chemotherapy. Although the underlying reasons are largely unknown, they might be as follows. Polymorphic variations in genes involved in drug metabolism are associated with the toxicity of platinum and fluoropyrimidine, which are the most common chemotherapeutic agents for gastric cancer. For example, the dihydropyridine dehydrogenase group of enzymes is responsible for the metabolism of fluoropyrimidines (25). Thirty-one single-nucleotide polymorphisms (SNPs) have been associated with a higher risk of docetaxel-induced neutropenia (26). Additional studies found an association between transporter-related SNPs and chemotherapy-induced neutropenia (27). With the same genetic polymorphisms, patients who develop toxicities from NAC are expected to be more likely to develop toxicities from adjuvant chemotherapy. Chemotherapy-induced neutropenia, a sign of potentially serious suppression of the host immune system, frequently leads to decreased relative dose intensity and poor compliance with treatment (28,29), and poor compliance correlates with adjuvant chemotherapy with inferior survival outcomes (30,31). Recent studies have correlated sarcopenia (low skeletal muscle mass) with an excess of chemotherapy toxicity (32), for which one reasonable explanation is the routine practice of body surface area-based dosing chemotherapy without considering that fat components comprise a large proportion of body weight. Moreover, this condition may worsen after surgery, chemotherapy or radiotherapy (33). Such sarcopenic patients may develop toxicities in postoperative chemotherapy, leading to poor compliance with postoperative therapy and ultimately inferior survival. Our findings also suggest that to avoid treatment discontinuation among patients with NISN, frequent surveillance of hematologic components and timely supportive treatments such as G-CSF are warranted to resolve chemotherapy toxicities.
The present analysis is certainly limited by its retrospective, non-randomized and monocentric design, and it is difficult to eliminate biases in selecting patients and documenting neutropenia events. Some toxicity events, especially less serious ones, may have been underreported. Second, the period of inclusion was long [2006–2016], and practices may have changed. Third, aiming to evaluate the relationship between NISN and pathological response, only patients who underwent surgical resection after NAC were eligible; thus, our conclusions cannot be applied to patients who failed to receive surgical resection. Finally, few patients had NISN during NAC, which limits the power of the statistical analyses. Multicentric prospective studies are warranted to validate these results.
Conclusions
In conclusion, our study revealed a link between NISN, pathological response, treatment compliance, and survival. Moreover, the prognostic role of NISN depends on postoperative chemotherapy. These data may help guide patient stratification and treatment strategy selection. Further prospective validation within multicentric studies is warranted to confirm the potential of neutropenia as a marker to individualize treatment strategies.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.68). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital has reviewed and approved this study, and has also agreed that individual patient consent was not required to report clinical outcomes alone (No. 17-156/1412).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Xu X, Zheng G, Zhang T, et al. Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother Pharmacol 2019;84:635-46. [Crossref] [PubMed]
- Smyth EC, Fassan M, Cunningham D, et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol 2016;34:2721-7. [Crossref] [PubMed]
- Noble F, Lloyd MA, Turkington R, et al. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br J Surg 2017;104:1816-28. [Crossref] [PubMed]
- Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur J Surg Oncol 2017;43:1607-16. [Crossref] [PubMed]
- Papaxoinis G, Kamposioras K, Weaver JMJ, et al. The Role of Continuing Perioperative Chemotherapy Post Surgery in Patients with Esophageal or Gastroesophageal Junction Adenocarcinoma: a Multicenter Cohort Study. J Gastrointest Surg 2019;23:1729-41. [Crossref] [PubMed]
- Saunders JH, Bowman CR, Reece-Smith AM, et al. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J Surg Oncol 2017;115:821-9. [Crossref] [PubMed]
- Klevebro F, Lindblad M, Johansson J, et al. Outcome of neoadjuvant therapies for cancer of the oesophagus or gastro-oesophageal junction based on a national data registry. Br J Surg 2016;103:1864-73. [Crossref] [PubMed]
- Wu C, Wang N, Zhou H, et al. Effects of Neoadjuvant Chemotherapy Toxicity and Postoperative Complications on Short-term and Long-term Outcomes After Curative Resection of Gastric Cancer. J Gastrointest Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Sunaga T, Suzuki S, Kogo M, et al. The association between neutropenia and prognosis in stage III colorectal cancer patients receiving adjuvant chemotherapy. Eur J Cancer Care (Engl) 2014;23:394-400. [Crossref] [PubMed]
- Hamauchi S, Yamazaki K, Masuishi T, et al. Neutropenia as a Predictive Factor in Metastatic Colorectal Cancer Treated With TAS-102. Clin Colorectal Cancer 2017;16:51-7. [Crossref] [PubMed]
- Konishi H, Fujiwara H, Shiozaki A, et al. Effects of neutropenia and histological responses in esophageal squamous cell carcinoma with neo-adjuvant chemotherapy. Int J Clin Oncol 2016;21:95-101. [Crossref] [PubMed]
- Diefenhardt M, Hofheinz RD, Martin D, et al. Leukocytosis and neutrophilia as independent prognostic immunological biomarkers for clinical outcome in the CAO/ARO/AIO-04 randomized phase 3 rectal cancer trial. Int J Cancer 2019;145:2282-91. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- National Cancer Institute. Common terminology criteria for adverse events v.3.0 and v.4.0 (CTCAE). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Published 2011.
- Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J Clin Oncol 2018;36:1443-53. [Crossref] [PubMed]
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3199-212. [Crossref] [PubMed]
- Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch 2018;472:175-86. [Crossref] [PubMed]
- Erpenbeck L, Schön MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene 2017;36:2483-90. [Crossref] [PubMed]
- Faget J, Groeneveld S, Boivin G, et al. Neutrophils and Snail Orchestrate the Establishment of a Pro-tumor Microenvironment in Lung Cancer. Cell Rep 2017;21:3190-204. [Crossref] [PubMed]
- Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016;150:1646-58.e17. [Crossref] [PubMed]
- Hatogai K, Fujii S, Kojima T, et al. Prognostic significance of tumor regression grade for patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy followed by surgery. J Surg Oncol 2016;113:390-6. [Crossref] [PubMed]
- Kadota T, Hatogai K, Yano T, et al. Pathological tumor regression grade of metastatic tumors in lymph node predicts prognosis in esophageal cancer patients. Cancer Sci 2018;109:2046-55. [Crossref] [PubMed]
- Cortejoso L, García-González X, García MI, et al. Cost-effectiveness of screening for DPYD polymorphisms to prevent neutropenia in cancer patients treated with fluoropyrimidines. Pharmacogenomics 2016;17:979-84. [Crossref] [PubMed]
- Nieuweboer AJM, Smid M, de Graan AJM, et al. Role of genetic variation in docetaxel-induced neutropenia and pharmacokinetics. Pharmacogenomics J 2016;16:519-24. [Crossref] [PubMed]
- Nieuweboer AJM, Smid M, de Graan AJM, et al. Predicting paclitaxel-induced neutropenia using the DMET platform. Pharmacogenomics 2015;16:1231-41. [Crossref] [PubMed]
- Lalami Y, Klastersky J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: An overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol 2017;120:163-79. [Crossref] [PubMed]
- Schraa SJ, Frerichs KA, Agterof MJ, et al. Relative dose intensity as a proxy measure of quality and prognosis in adjuvant chemotherapy for breast cancer in daily clinical practice. Eur J Cancer 2017;79:152-7. [Crossref] [PubMed]
- Yamashita K, Kurokawa Y, Yamamoto K, et al. Risk Factors for Poor Compliance with Adjuvant S-1 Chemotherapy for Gastric Cancer: A Multicenter Retrospective Study. Ann Surg Oncol 2017;24:2639-45. [Crossref] [PubMed]
- Jang SH, Jung YJ, Kim MG, et al. The prognostic significance of compliance with postoperative adjuvant chemotherapy in patients with stage III gastric cancer: An observational study. J Gastric Cancer 2018;18:48-57. [Crossref] [PubMed]
- Shachar SS, Deal AM, Weinberg M, et al. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin Cancer Res 2017;23:658-65. [Crossref] [PubMed]
- Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107-18. [Crossref] [PubMed]