
Differences in lower cranial nerve complications predicted by the NTCP model between RTOG and reduced-volume IMRT planning in radiotherapy for nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) has a high disease incidence in China, and radiotherapy is the primary treatment modality for nonmetastatic NPC patients (1,2). With the application of the intensity-modulated radiotherapy (IMRT) technique, the majority of patients can be cured and have a long-term survival time; thus, reducing the radiation side effects of organs at risk (OARs) to improve the quality of life of patients is particularly important.
Among all known long-term side effects, radiation-induced cranial nerve palsy (RICNP) is not a rare complication; it usually occurs in the lower cranial nerves (LCNs), with an incidence ranging from 0.3% to 20.6% (3-10). RICNP in NPC significantly compromises the patients’ quality of life and can even endanger their lives. Damage to the glossopharyngeal nerve (IX) causes loss of sensation in the pharynx and decreases salivation. Palsy of the vagus nerve (X) leads to impaired parasympathetic functions of almost all organs, and palsy of the hypoglossal nerve (XII) causes complete paralysis of the ipsilateral side of the tongue (11). However, LCNs have not served as a conventional OAR when delineating radiotherapy for head and neck cancer, including NPC. One of the reasons may be that the LCNs adjacent to carotid sheath are included in the high-dose irradiation volume. Moreover, the doses of radiation that LCNs are exposed to in the Radiation Therapy Oncology Group (RTOG)-guided IMRT plan (RTOG plan) are difficult to adjust to decrease the possibility of complication development. The reduced-volume IMRT plan (reduced-volume plan) has been used in recent years in the treatment of NPC, and the efficacy of this technique is comparable to that of the RTOG plan (12-14). The application of reduced-volume IMRT may provide the opportunity to decrease the doses received by LCNs. However, few studies have assessed the risk of LCN complications in NPC patients after reduced-volume IMRT. Moreover, the difference in LCN complications between RTOG and reduced-volume IMRT remain unknown. In the present study, therefore, we evaluated the difference in LCN complications predicted by the normal tissue complication probability (NTCP) model between RTOG and reduced-volume IMRT planning in radiotherapy for NPC.
Methods
Patient characteristics
From May 2013 to September 2017, 50 patients with newly diagnosed NPC receiving curative radiotherapy were retrieved, and the characteristics are summarized in Table 1. All patients underwent disease staging according to the American Joint Committee on Cancer staging system [2002] and were divided into two T-stage groups, 25 patients in the T1-2 group and 25 patients in the T3-4 group. All patients had no LCN palsy on physical examination before radiotherapy.
Table 1
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 31 | 62.0 |
| Female | 19 | 38.0 |
| Age (y) | ||
| Range | 18–85 | |
| Median | 58.5 | |
| T-stage | ||
| T1 | 6 | 12.0 |
| T2 | 19 | 38.0 |
| T3 | 14 | 28.0 |
| T4 | 11 | 22.0 |
| N-stage | ||
| N0 | 6 | 12.0 |
| N1 | 8 | 16.0 |
| N2 | 27 | 54.0 |
| N3 | 9 | 18.0 |
CT-sim scanning and target volume delineation
All patients were positioned and immobilized from the head to the shoulder by a thermoplastic mask. Computed tomography (CT) images with a 3-mm slice thickness of the head and neck region were obtained and imported into the treatment planning system. The target of each patient was contoured according to RTOG 0225 clinical trial (15,16) and reduced-volume IMRT recommendations (12,13,17). For the RTOG-guided definition, the target volume included the gross tumor volume (GTV), the clinical target volume (CTV), and the planning target volume (PTV). The GTVnx and GTVnd covered the visible primary tumor and neck metastatic lymph nodes shown on the CT/MRI image, respectively. CTV1 encompassed high-risk structures surrounding the primary tumor, CTV2 covered the high-risk neck region, and CTV3 encompassed the low-risk neck region. PTVnx, PTVnd, PTV1, PTV2, and PTV3 consisted of a 3-mm margin in all directions around the corresponding GTV and CTV, respectively. The OARs included the brain stem, spinal cord, parotid glands, lenses, eyes, optic nerves, chiasm, cochlea, mandible, oral cavity and larynx. For reduced-volume IMRT recommendation, the definition of the target volume was the same as that defined in the protocol of RTOG 0225 clinical trial, except for CTV1 contouring. CTV1 was separated into two irradiation dose gradients, including CTV1-h, which encompassed both a 5-mm margin in all directions around the corresponding GTVnx and a 5-mm submucosa of the whole nasopharynx. PTV1-h consisted of a 3-mm margin in all directions around the CTV1-h. The OARs included the brain stem, spinal cord, parotid glands, lenses, eyes, optic nerves, chiasm, cochlea, mandible, oral cavity and larynx.
LCN delineation
The bilateral LCNs were delineated following the guidance provided by Mourad et al. (18). All delineations were based on the CT image of contrast medium enhancement, and scanning was performed with a 3-mm slice thickness. A CT window width of 1,400 and a window level of 400 were selected for delineation of the jugular foramen (JF) and the hypoglossal canal, while a window width of 180 and a window level of 950 were selected for delineation of the internal carotid artery (ICA).
Due to the anatomical nature and proximity of cranial nerves X and XI, they were contoured and grouped as one structure, while cranial nerve XII was contoured individually. Cranial nerves IX and XI emerge from the lateral aspect of the medulla oblongata and pass through the JF. After their exit from the JF, they pass vertically down the neck within the carotid sheath, lying between the internal jugular vein (IJV) and the ICA to the upper border of the thyroid cartilage (i.e., at the level of the lower border of C4). First, bilateral JFs and carotid sheaths, such as the IX, X, and XI cranial nerves, were delineated. The carotid sheath was formed based on delineation of the carotid artery with a 3-mm uniform margin, while the carotid artery was contoured from the inferior JF to the carotid bifurcation. Second, bilateral hypoglossal canals and corresponding carotid sheaths, such as the XII cranial nerve, were contoured. Finally, bilateral merging parts of the IX, X, and XI cranial nerves were delineated. One case of LCN contour is illustrated in Figure 1.
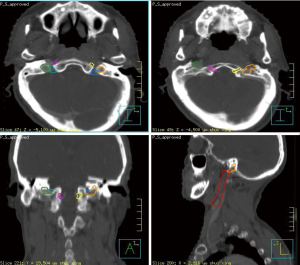
Treatment planning
The Philips Pinnacle Planning System 9.0 was used for IMRT planning. The treatment plan was designed for all patients with a standard coplanar 9-field gantry arrangement and delivered using a Siemens Primus Linac equipped with a 58-leaf MLC. A direct machine parameter optimization (DMPO) module was adopted for treatment planning. The maximum number of segments was set to 80, the minimum segment area was 5 cm2, and the minimum monitor unit (MU) was 5 MUs. A collapsed-cone convolution algorithm was used to calculate dosage with a dose grid resolution of 3 mm. According to the planning of the RTOG 0225 clinical trial, the prescribed dose was as follows: 6,600–7,040 cGy to the PTVnxd and PTVnd in 32 fractions, 6,000 cGy to the PTV1 and PTV2 in 30–32 fractions, and 5,400 cGy to the PTV3 in 30–32 fractions. For the planning of reduced-volume IMRT recommendations, the prescribed dose was the same as given in the RTOG-0225 guidelines, except for CTV1. The prescribed dose included 6,000 cGy to the PTV1-h in 30–32 fractions and 5,400 cGy to the PTV1 in 30 fractions.
The treatment goals for the IMRT plan were that the prescribed dose would cover 95% of the PTV volume, and the maximum dose would not exceed 107%. Regarding the OARs, the maximum doses to the brain stem and the spinal cord were set to 5,400 and 4,500 cGy, respectively. In addition, the doses to other normal tissues was minimized within a reasonable range without affecting the target coverage.
The incidence of LCN complications predicted by the NTCP model
The maximum dose (Dmax) and the mean dose (Dmean) of bilateral LCNs from RTOG and reduced-volume IMRT planning were investigated in each patient. The Lyman-Kutcher-Burman (LKB) calculation model in Pinnacle3 was used to predict the incidence of LCN complications (19-21). Because the characteristics of LCNs are similar to those of the optic nerve, the parameters applied in the present study were calculated based on the optic nerve as follows: α/β =3 and the TD50 (the mean dose predicting a 50% risk of complications), n (a parameter that considers the volume effect), and m (the slope of the dose–response curve) were 6,500 cGy, 0.25, and 0.14, respectively (20,22,23).
Statistical analysis
SPSS 19.0 software was used for data analysis, and Sigma Plot 10.0 was used for figure plotting. A paired sample t-test was used to compare the differences in all considered parameters between the RTOG and reduced-volume IMRT planning approaches and between the clinical T1-2 and T3-4 groups. The correlations between LCN NTCP and dose volume were analyzed using Pearson’s correlation coefficient. A two-tailed value of P<0.05 was considered statistically significant.
Results
Volume of LCNs and volume ratio included in the target and dose
The LCN volume of 50 patients was (10.07±1.51) cc, 25.73%±15.92% of which was included in PTVnx and PTVnd and 96.42%±8.57% of which was included in PTV1 and PTV2. The ratios of LCNs included in doses of more than 5,400, 6,000 and 6,600 cGy are shown in Table 2. The ratios in the RTOG plans were all significantly higher than those in the reduced-volume plans.
Table 2
| Dose (cGy) | Patients | RTOG (%) | Reduced volume (%) | t | P |
|---|---|---|---|---|---|
| ≥6,600 | Total_Pat | 61.48±26.03 | 47.26±23.23 | 6.16 | 0.000 |
| T1-2 | 53.12±28.85 | 37.29±21.88 | 3.76 | 0.000 | |
| T3-4 | 69.84±20.15 | 57.23±20.40 | 6.38 | 0.000 | |
| ≥6,000 | Total_Pat | 93.13±11.68 | 64.95±21.78 | 9.49 | 0.000 |
| T1-2 | 91.86±11.07 | 57.79±21.42 | 7.48 | 0.000 | |
| T3-4 | 94.40±12.36 | 72.12±20.05 | 6.35 | 0.000 | |
| ≥5,400 | Total_Pat | 99.40±0.79 | 98.58±1.25 | 7.18 | 0.000 |
| T1-2 | 99.29±0.69 | 98.17±1.29 | 5.98 | 0.000 | |
| T3-4 | 99.51±0.88 | 98.98±1.09 | 4.95 | 0.000 |
Total_Pat, total patients; RTOG and reduced volume were the treatment plans based on the IMRT recommendations of RTOG and reduced volume, respectively; t, P, difference between the two plans. LCN, lower cranial nerve.
Dosimetry of LCNs in different treatment plans
One case of planning and the dose volume histogram of LCNs is shown in Figures 2,3. Dosimetric differences of LCNs in two different treatment plans and different clinical T stages are shown in Table 3. The Dmax and Dmean of LCNs in RTOG plans were significantly larger than those in reduced-volume plans [(7,453±170) vs. (7,401±148) cGy (t=3.01, P=0.004), (6,740±279) vs. (6,436±375) cGy (t=9.86, P=0.000)]. Furthermore, the Dmax and the Dmean of LCNs in patients with clinical T1-2 stages were significantly lower than in patients with clinical T3-4 stages [(7,390±187) vs. (7,464±119) cGy (t=−2.40, P=0.019), (6,442±338) vs. (6,733±329) cGy (t=−4.78, P=0.000)].
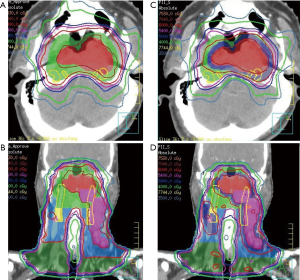
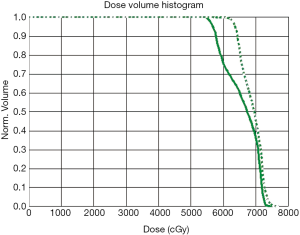
Table 3
| Variable | Patients | RTOG | Reduced volume | t | P |
|---|---|---|---|---|---|
| Dmax (cGy) | Total_Pat | 7,453±170 | 7,401±148 | 3.01 | 0.004 |
| T1-2 | 7,425±195 | 7,355±177 | 2.53 | 0.018 | |
| T3-4 | 7,481±139 | 7,447±95 | 1.64 | 0.113 | |
| Dmean (cGy) | Total_Pat | 6,740±279 | 6,436±375 | 9.86 | 0.000 |
| T1-2 | 6,622±231 | 6,263±336 | 6.70 | 0.000 | |
| T3-4 | 6,858±277 | 6,609±334 | 8.99 | 0.000 | |
| NTCP (%) | Total_Pat | 59.98±11.87 | 51.62±13.86 | 8.81 | 0.000 |
| T1-2 | 56.56±9.70 | 46.88±11.61 | 6.30 | 0.000 | |
| T3-4 | 63.40±13.00 | 56.36±14.52 | 6.50 | 0.000 |
Dmax, maximum point dose; Dmean, mean dose; Total_Pat, total patients; RTOG and reduced volume were the treatment plans based on IMRT recommendations of RTOG and reduced volume, respectively; t, P, difference between the two plans. LCN, lower cranial nerve; NTCP, normal tissue complication probability.
The incidence of LCN complications predicted by the NTCP model in different treatment plans
As shown in Table 3, the NTCP in RTOG plans was significantly higher than that in the reduced-volume plans [(59.98±11.87)% vs. (51.62±13.86)%, P=0.000], and NTCP was significantly lower in the T1-2 group than in the T3-4 group [(51.72±11.66)% vs. (59.88±14.10)%, t=−3.14, P=0.002)].
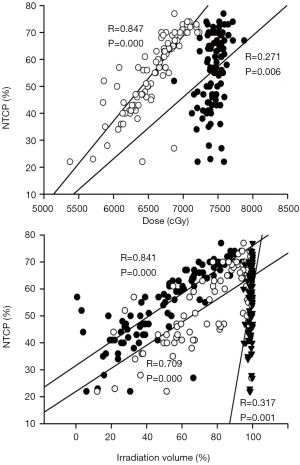
As shown in Figure 4, there was an extremely strong correlation of NTCP with Dmean (R=0.847, P=0.000) and a weak correlation with Dmax (R=0.271, P=0.006). Similarly, there was a strong correlation of NTCP with an irradiation volume of more than 6,600 cGy (R=0.841, P=0.000), a moderate correlation with 6,000 cGy (R=0.709, P=0.000), and a weak correlation with 5,400 cGy (R=0.317, P=0.001).
Discussion
RICNP is not a rare complication and can be a long-term problem after radical radiotherapy for NPC. Prevention of RICNP is paramount because the functional impairment can be profound and refractory to standard therapies.
Several risk factors for RICNP have been described. Kong et al. identified initial CNP at diagnosis, chemotherapy, total radiation dose and upper neck fibrosis as independent risk factors for developing RICNP (24). However, the majority of studies on RICNP included patients prior to the availability of IMRT, which improved the therapeutic ratio and/or treatment tolerance in patients with head and neck cancer by facilitating the sparing of normal tissue, and IMRT is already the standard treatment for NPC. The potential overlap among fields irradiated in the neck is often the main cause of RICNP in conventional radiotherapy, while RICNP generally does not appear in IMRT, and neck fibrosis is also expected to significantly decrease with the use of IMRT treatment given the dose restraint to posterior neck muscles. However, the effects of dose escalation in IMRT may give rise to the long-term development of CNP (24). Instead of being unable to accurately locate the LCNs in conventional radiotherapy, the precise delineation of LCNs is now possible in IMRT planning with the application of CT and MRI techniques. Dose evaluation and NTCP prediction are also available.
The reduced-volume plan was an expert consensus provided by the Working Committee for the clinical staging of NPC in China in 2010, in which the CTV is reduced accordingly on the basis of RTOG 0225 clinical trial; its long-term curative effect has also been confirmed to be ideal (12-14). However, theoretically, there could also be reduced toxicity to surrounding OARs. The reduced-volume plan and the RTOG plan are the two most commonly used IMRT plans in China today. The purpose of this study was to evaluate the difference in LCN NTCP between the two IMRT plans.
The results showed that the Dmax and the Dmean of LCNs in the reduced-volume plan were significantly lower than those in the RTOG plan (7,401 vs. 7,453 cGy, 6,436 vs. 6,740 cGy); NTCP was also significantly lower in the reduced-volume plan (51.62% vs. 59.98%). On the other hand, the Dmax, the Dmean and NTCP of LCNs in the T1-2 group were significantly lower than those in the T3-4 group (7,390 vs. 7,464 cGy, 6,442 vs. 6,733 cGy, 51.72% vs. 59.88%). Therefore, our results showed that LCNs can be better spared in T1-2 stage patients with a reduced-volume plan.
RICNP is related to many factors. A total of 512 NPC cases were analyzed retrospectively by Kong et al. (25). Cranial nerve injury developed in 81 of 512 cases, and the 5- and 10-year cumulative incidence rates were 10.3% and 25.4%, respectively. Injury to the XII cranial nerves was most common. Multivariate analysis showed that RICNP was mainly associated with tumor invasion, chemotherapy, nasopharyngeal total radiation dose and age, while LCN injury was associated with N staging and radiation field. LCN injury increased for later stages of cervical lymph node. Thirty-one patients with breast cancer were selected by Wu et al. (26), and 50 Gy of postoperative adjuvant radiotherapy was delivered to the ipsilateral supraclavicular area and chest wall. Dmax, Dmean, V40, V45, V50, V52.5, and V55 of BP were evaluated. The results showed that there was no relationship between BP neuropathy and Dmax, but the number of lymph node dissections was an independent influencing factor of BP lesions.
In fact, few studies have been reported on LCN injury after radiotherapy in NPC, and even fewer studies on NTCP are available. There is currently a lack of corresponding parameters of NTCP in LCN injury. Because LCNs have characteristics similar to those of the optic nerve, parameters of the optic nerve (TD50, n and m: 6,500 cGy, 0.25, and 0.14) from Burman (20) were used in this study to calculate the NTCP.
In our results, there exists strong correlations of LCN NTCP with Dmean (R=0.847, P=0.000) and an irradiation volume of more than 6,600 cGy (R=0.841, P=0.000), while there is a weak correlation with Dmax (R=0.271, P=0.006). Though LCNs are serial organs, the irradiation condition of the maximum point dose to the LCN could vary. Sometimes only a tiny volume of the LCN was exposed to the Dmax, while in rest of the LCN volume was exposed to relatively lower doses; thus, the Dmax may not truly reflect the LCN dose. As illustrated in Figure 4, NTCP increased with increasing Dmean and for an irradiation volume of more than 6,000 cGy, but NTCP did not increase with Dmax. On the other hand, the higher NTCP in the T3-T4 group may be related to the high irradiation volume of more than 6,600 cGy to the PTVnx and PTVnd (as shown in Tables 2,3). In addition, previous studies revealed that LCN injury was related to both a high dose and the irradiated volume, which induced severe fibrosis on the carotid sheath (25,27).
Conclusions
It is feasible to precisely delineate the LCN, which can serve as a routine OAR, and to further predict LCN complications using the NTCP model in IMRT planning for NPC. Both a high Dmean and a large irradiation volume are important factors in predicting complications of LCNs. Of the two most common IMRT guidance plans in China, LCN NTCP was significantly lower for the reduced-volume plan than for the RTOG plan.
Acknowledgments
We thank American Journal Experts for assisting in the preparation of this manuscript.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.75). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The Second Affiliated Hospital of Soochow University (No. 2015021). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chow JCH, Cheung KM, Au KH, et al. Radiation-induced hypoglossal nerve palsy after definitive radiotherapy for nasopharyngeal carcinoma: Clinical predictors and dose–toxicity relationship. Radiother Oncol 2019;138:93-8. [Crossref] [PubMed]
- Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 2018;77:16-21. [Crossref] [PubMed]
- Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976-1985: late complications following megavoltage irradiation. Brit J Radiol 1992;65:918-28. [Crossref] [PubMed]
- Fu KK, Pajak TF, Trotti A, et al. A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000;48:7-16. [Crossref] [PubMed]
- Hutcheson KA, Yuk M, Hubbard R, et al. Delayed Lower Cranial Neuropathy after Oropharyngeal IMRT: A Cohort Analysis and Literature Review. Head Neck 2017;39:1516-23. [Crossref] [PubMed]
- Aggarwal P, Zaveri JS, Goepfert RP, et al. Symptom Burden Associated With Late Lower Cranial Neuropathy in Long-term Oropharyngeal Cancer Survivors. JAMA Otolaryngol Head Neck Surg 2018;144:1066-76. [Crossref] [PubMed]
- Zheng Y, Han F, Xiao W, et al. Analysis of late toxicity in nasopharyngeal carcinoma patients treated with intensity modulated radiation therapy. Radiat Oncol 2015;10:17. [Crossref] [PubMed]
- Luk YS, Shum JS, Sze HC, et al. Predictive factors and radiological features of radiation-induced cranial nerve palsy in patients with nasopharyngeal carcinoma following radical radiotherapy. Oral Oncol 2013;49:49-54. [Crossref] [PubMed]
- McDowell LJ, Kathy R, Wei X, et al. Long-Term Late Toxicity, Quality of Life, and Emotional Distress in Patients With Nasopharyngeal Carcinoma Treated With Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys 2018;102:340-52. [Crossref] [PubMed]
- Huang TL, Chien CY, Tsai WL, et al. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck 2016;38:E1026-32. [Crossref] [PubMed]
- Janssen S, Glanzmann C, Yousefi B, et al. Radiation-induced lower cranial nerve palsy in patients with head and neck carcinoma. Mol Clin Oncol 2015;3:811-6. [Crossref] [PubMed]
- Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys 2009;75:1071-8. [Crossref] [PubMed]
- Pan JJ, Han L, Zhang Y, et al. Impact of reducing clinical target volume on efficacy of intensity modulated radiation therapy for nasopharyngeal carcinoma. Chin J Radiat Oncol 2010;19:283-7.
- Lin SJ, Pan JJ, Han L, et al. Long-term outcome of nasopharyngeal carcinoma treated with reduced-volume intensity-Modulated radiotherapy and chemotherapy. Chin J Radiat Oncol 2013;22:378-81.
- Lee NY, Harris J, Garden A, et al. Phase II Multi-Institutional Study of IMRT ± Chemotherapy for Nasopharyngeal Carcinoma (RTOG 0225): Preliminary Results. Int J Radiat Oncol Biol Phys 2007;69:S13-4. [Crossref]
- Lee NY, Qiang Z, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol 2012;13:172-80. [Crossref] [PubMed]
- Working committee for the clinical stage of nasopharyngeal carcinoma in China. Expert consensus on IMRT targets and doses designing for nasopharyngeal carcinoma patients in 2010. Chin J Radiat Oncol 2011;20:267-9.
- Mourad WF, Young BM, Young R, et al. Clinical validation and applications for CT-based atlas for contouring the lower cranial nerves for head and neck cancer radiation therapy. Oral Oncol 2013;49:956-63. [Crossref] [PubMed]
- Webb S, Nahum AE. A model for calculating tumor control probability in radiotherapy including the effects of inhomogeneous disributions of dose and clonogenic cell density. Phys Med Biol 1993;38:653-66. [Crossref] [PubMed]
- Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123-35. [Crossref] [PubMed]
- Lyman JT, Wolbarst AB. Optimization of radiation therapy III: a method of assessing complication probabilities from dose volume histograms. Int J Radiat Oncol Biol Phys 1987;13:103-9. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Pan JJ, Hong JS, Zhang Y, et al. Dosimetric Study of lower cranial nerve after conventional radiotherapy in patients with Nasopharyngeal carcinoma. J Fujian Med Univ 2006;40:132-5.
- Kong L, Lu JJ, Liss AL, et al. Radiation induced cranial nerve palsy: A cross sectional study of nasopharyngeal cancer patients after definitive radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:1421-7. [Crossref] [PubMed]
- Kong L, Zhang YW, Wu YR, et al. Radiation-induced cranial nerve palsy and its causative factors in Nasopharyngeal carcinoma. Chin J Radiat Oncol 2005;14:10-4.
- Wu SG, Huang SJ, Zhou J, et al. Dosimetric analysis of the brachial plexus among patients with breast cancer treated with post-mastectomy radiotherapy to the ipsilateral supraclavicular area: report of 3 cases of radiation-induced brachial plexus neuropathy. Radiat Oncol 2014;9:292. [Crossref] [PubMed]
- Wei BQ. Radiation induced cranial neuropathy (RICN) after radiotherapy for Nasopharyngeal carcinoma (NPC): Cause related to radiation technique. Chin J Radiat Oncol 1994;3:164-5.


