
C-X-C motif chemokine receptor type 2 correlates with higher disease stages and predicts worse prognosis, and its downregulation enhances chemotherapy sensitivity in triple-negative breast cancer
Introduction
Breast cancer is the most common female cancer as well as the leading cause of cancer-related death among females worldwide, which leads to approximately 1,700,000 new cases and 520,000 deaths globally according to cancer statistics at 2018 (1,2). Up to 10–15% of breast cancer cases do not express either estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), which are known as triple-negative breast cancer (TNBC) (3). Consequently, TNBC is difficult to benefit from common therapies due to lack of well-defined treatment targets. Chemotherapy is chosen as a standard systemic treatment for 90% of TNBC patients, whereas resistance to chemotherapy as well as tumor relapse and metastases are more prevalent in TNBC patients than in other subtypes, which are all correlated with poor prognosis (3-6). Thus, it is urgent to explore novel biomarkers for disease progression and prognosis in TNBC, which may contribute to establishing suitable individual therapies.
Chemokines, which are small (6−14 kDa) secreted proteins, are essential in the immune system by mediating the activation of immune cells during innate and adaptive responses (7). During the last decade, chemokines have become a focus as biomarkers for tumor inhibition strategies (8,9). C-X-C motif chemokine receptor type 2 (CXCR2) is an important receptor that binds multiple chemokines, such as C-X-C chemokine ligand (CXCL) 1, 2, 3, 5, 6, 7 and 8 (10). CXCR2 is known to be enriched in monocytes, granulocytes, mast cells and some natural killer cells, and it has been found overexpressed in several cancers recently (8). Moreover, CXCR2 has been reported to correlate with aggressive disease progression (such as larger tumor size, deeper tumor invasion and increased TNM stage) and poor prognosis in several cancers including breast cancer (11,12). Furthermore, a previous study reveals that targeting CXCR2 contributes to circumvent chemotherapy resistance in breast cancer, suggesting the involvement of CXCR2 in chemotherapy resistance (13). Given that CXCR2 facilitates disease progression and chemotherapy resistance in various cancers including breast cancer, and it presents with promotive effect on chemotherapy resistance of breast cancer, we assumed that CXCR2 might also be involved in the disease progression, prognosis and drug resistance in TNBC, while related research was seldomly reported.
Therefore, we conducted this study to explore the correlation of CXCR2 expression with tumor stage and overall survival (OS) in TNBC patients, furthermore, to investigate the influence of CXCR2 downregulation on chemotherapy sensitivity in TNBC cells.
Methods
Patients
A total of 158 TNBC patients who underwent surgical excision in our hospital between January 2007 and December 2011 were reviewed in this retrospective study. The inclusion criteria were: (I) diagnosed as primary breast cancer by histopathological examination; (II) ER, PR and human epidermal growth factor receptor-2 (HER2) were negative; (III) TNM stage I-III and underwent surgical excision; (IV) clinical data and follow-up record were complete; (V) the tumor tissue specimens which were taken from surgical excision or needle biopsy were well preserved and available for immunohistochemistry (IHC) assay. The exclusion criteria were: (I) previous breast surgical operation; (II) history of serious infection (e.g., human immunodeficiency virus) or other malignancies. This study was approved by Ethics Committee of our hospital, and all patients or their family members provided written informed consents or verbal agreements with recording.
Data collection
The basic clinical data were collected from medical record including age, tumor size, number of positive lymph nodes, pathological grade, T stage, N stage and TNM stage. The survival data were obtained from follow-up record, and the median follow-up duration was 99.5 months ranging from 2.0–149.0 months. OS was measured from the date of surgical excision to the date of death, and patients not known to have died at last follow-up were censored on the date they were last known to be alive.
IHC
The specimens of tumor tissue were acquired from storage compartment after approval from the pathology department of our hospital. For the patients who did not receive neoadjuvant chemotherapy, the tumor tissue specimens were obtained from surgical excision, and for the patients who received neoadjuvant chemotherapy, the tumor tissue specimens were collected from needle biopsy before neoadjuvant chemotherapy. All the tissues were formalin-fixed paraffin-embedded. The tissue specimens were cut into 4 µm sections, deparaffinized in xylene (Sigma-Aldrich, USA) and rehydrated in graded ethanol. Then, the tissue sections were rinsed with 0.025% Triton X-100 tris buffered saline (TBS) (Sigma-Aldrich, USA). Antigen retrieval was performed by microwave heating and endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide (Sigma-Aldrich, USA) for 15 min. Then, the tissue sections were incubated with rabbit Anti-CXCR2 antibody (ab14935) (Abcam, USA) at 4 °C overnight, and blocked with 10% goat serum (Thermo Fisher, USA) for 60 min at room temperature. After that, the tissue sections were incubated with horseradish peroxidase (HRP)-conjugated goat-anti-rabbit immunoglobulin G antibody (Abcam, USA) for 30 min at 37 °C. Staining of the tissue sections was conducted using 3,3’-diaminobenzidine (DAB) (Dako, USA), and counterstaining of the tissue sections was performed with hematoxylin (Sigma-Aldrich, USA). Finally, the tissue sections were gently cleaned in water for 10 min and then sealed with a neutral resin (Sango Biotech, China), and observed under a BX41 microscope (Olympus, Japan).
IHC analysis
The expression of CXCR2 in tumor tissue was assessed by a semiquantitative scoring method according to the previously reported methodology (14). The immunostaining intensity and the percentage of positive cells were assessed and scored. The staining intensity score was based on four classes: 0 (no staining); 1 (week staining); 2 (moderate staining); 3 (intense staining). Positive cell was identified by the colored cytoplasm and membrane of the tumor cell in IHC staining. The percentage of positive tumor cells was classified into five grades, as follows: 0 (negative); 1 (≤25%); 2 (26–50%); 3 (51–75%); 4 (≥76%). Finally, the total score of IHC staining was obtained by multiplying the staining intensity score and percentage scores. Sections with a total score ≥3 were defined as high expression of CXCR2, and scores <3 were defined as low expression of CXCR2.
Cell culture and transfection
Human TNBC cell line HCC1937 was purchased from American Type Culture Collection (ATCC) (Rockefeller, USA), and then cultured in 90% RPMI-1640 Medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) at 37 °C under 95% air and 5% CO2 condition. CXCR2 shRNA and control shRNA were established by Shanghai GenePharma Bio-tech Company (Shanghai, China) and transfected into HCC1937 cells, named as CXCR2 (-) group and negative control (-) [NC (-)] group, respectively.
Chemotherapy sensitivity
After 48 h of transfection, doxorubicin with different concentrations (0, 4, 8, 16, 32, 64 and 128 nM), docetaxel with different concentrations (0, 1, 2, 4, 8, 16 and 32 nM) were used to treat HCC1937 cells. The setting of chemotherapy drug concentrations was referred to a previous study (15). Subsequently, after being cultured for additional 48 h, cell viability was measured using Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s instructions. Finally, the relative cell viability (%) was calculated by setting corresponding untreated controls (0 µM drug) as 100%, and IC50 of each drug was calculated.
Statistical analysis
Statistical analyses were performed with the use of SPSS 24.0 software (IBM, USA), and figures were plotted using GraphPad Prism 7.00 software (GraphPad Software, USA). Continuous variable was displayed as mean ± standard deviation (SD) and categorized variable was expressed as count (percentage). The correlation of CXCR2 expression with pathological grade, T stage, N stage and TNM stage was determined by Wilcoxon rank sum test. OS was displayed by Kaplan-Meier curve, and the difference of OS between CXCR2 high and low expression patients was determined by log-rank test. The factors affecting OS were analyzed by univariate and multivariate Cox’s proportional hazard regression model. As for cell experiments, comparison between two groups was determined by t-test, and IC50 was calculated using Probit regression. All tests were 2-sided, and P value <0.05 was considered as significant.
Results
Clinical characteristics
One fifty-eight TNBC patients with mean age of 52.5±12.8 years were enrolled in this study (Table 1). The mean tumor size was 4.0±3.1 cm, meanwhile, the number of positive lymph nodes was 3.0±3.7. As for disease stage, patients with pathological grade G1, G2 and G3 were 30 (19.0%), 117 (74.0%) and 11 (7.0%) respectively; patients with T1, T2 and T3 stage were 50 (31.7%), 77 (48.7%) and 31 (19.6%) respectively; patients with N0, N1, N2 and N3 stage were 43 (27.2%), 60 (38.0%), 37 (23.4%) and 18 (11.4%) respectively; patients with TNM stage I, II and III were 14 (8.9%), 89 (56.3%) and 55 (34.8%) respectively.
Table 1
| Items | TNBC patients (N=158) |
|---|---|
| Age (years), mean ± SD | 52.5±12.8 |
| Tumor size (cm), mean ± SD | 4.0±3.1 |
| Number of positive lymph nodes, mean ± SD | 3.0±3.7 |
| Pathological grade, N (%) | |
| G1 | 30 (19.0) |
| G2 | 117 (74.0) |
| G3 | 11 (7.0) |
| T stage, N (%) | |
| T1 | 50 (31.7) |
| T2 | 77 (48.7) |
| T3 | 31 (19.6) |
| N stage, N (%) | |
| N0 | 43 (27.2) |
| N1 | 60 (38.0) |
| N2 | 37 (23.4) |
| N3 | 18 (11.4) |
| TNM stage, N (%) | |
| I | 14 (8.9) |
| II | 89 (56.3) |
| III | 55 (34.8) |
TNBC, triple negative breast cancer; SD, standard deviation.
CXCR2 expression in tumor tissues
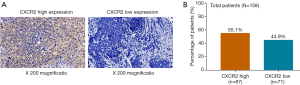
CXCR2 high expression and low expression detected by IHC analysis were exhibited in Figure 1A. According to the total score of IHC staining, samples were classified as CXCR2 high expression and CXCR2 low expression, and there were 87 (55.1%) patients presented with CXCR2 high expression and 71 (44.9%) patients presented with CXCR2 low expression (Figure 1B).
Correlation of CXCR2 expression with tumor stages
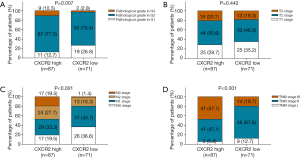
To evaluate the correlation of CXCR2 expression with clinical features, we analyzed CXCR2 expression in TNBC patients with various tumor stages. For pathological grade, CXCR2 high expression was associated with increased pathological grade (P=0.007) (Figure 2A). In addition, CXCR2 high expression was associated with elevated N stage (P<0.001) (Figure 2B) as well as TNM stage (P<0.0.001) (Figure 2C), while no correlation of CXCR2 expression with T stage (P=0.422) was found (Figure 2D).
Comparison of OS between CXCR2 high expression patients and CXCR2 low expression patients
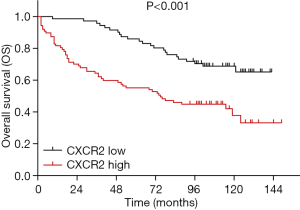
K-M curves disclosed that OS in TNBC patients with CXCR2 high expression was dramatically shorter than that in patients with CXCR2 low expression patients (P<0.001) (Figure 3).
Analysis of factors predicting OS
Univariate Cox’s regression analysis showed that CXCR2 high expression (P<0.001) was associated with worse OS in TNBC patients, meanwhile, higher pathological grade (P<0.001) and higher N stage (P=0.001) correlated with unfavorable OS as well (Table 2). Furthermore, multivariate Cox’s regression analysis disclosed that CXCR2 high expression was an independent predictive factor for decreased OS (P=0.028), and age (>50 years) (P<0.001), higher pathological grade (P<0.001) as well as higher N stage (P<0.001) also independently predicted worse OS in TNBC patients.
Table 2
| Items | Univariate Cox’s regression | Multivariate Cox’s regression | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| CXCR2 high expression | <0.001 | 2.596 (1.583–4.257) | 0.028 | 1.846 (1.067–3.194) | |
| Age (>50 years) | 0.734 | 1.084 (0.680–1.730) | <0.001 | 3.644 (1.806–7.353) | |
| Higher pathological grade | <0.001 | 2.845 (1.750–4.625) | <0.001 | 3.129 (1.921–5.097) | |
| Higher T stage | 0.313 | 0.837 (0.593–1.182) | 0.371 | 0.820 (0.532–1.266) | |
| Higher N stage | 0.001 | 1.486 (1.185–1.864) | <0.001 | 2.139 (1.593–2.873) | |
OS, overall survival; HR, hazard ratio; CI, confidence; CXCR2, C-X-C motif chemokine receptor type 2.
Effect of CXCR2 downregulation on chemotherapy sensitivity
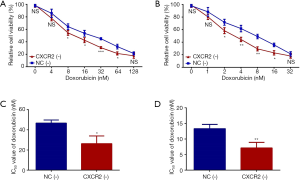
To further evaluate whether CXCR2 correlated with TNBC prognosis via affecting chemotherapy sensitivity, we assessed the relative cell viability (%) of HCC1937 cells treated by doxorubicin and docetaxel with different concentrations after CXCR2 knockdown, and further calculated the IC50 value of each drug. The relative cell viability was reduced in CXCR2 (-) group compared to NC (-) group when treated by doxorubicin at concentration of 8 nM (P<0.05), 16 nM (P<0.05), 32 nM (P<0.001) and 64 nM (P<0.05) (Figure 4A), but was similar between the two groups at doxorubicin concentration of 4 and 128 nM (all P value >0.05). Besides, the relative cell viability was decreased in CXCR2 (-) group compared to NC (-) group when treated by docetaxel at the concentration of 2 nM (P<0.05), 4 nM (P<0.01), 8 nM (P<0.01) and 16 nM (P<0.05) (Figure 4B), but was similar between the two groups at docetaxel concentration of 1 and 32 nM (all P value >0.05). Moreover, IC50 value of doxorubicin (P<0.05) (Figure 4C) as well as IC50 value of docetaxel (P<0.01) (Figure 4D) were lower in CXCR2 (-) group compared to NC (-) group. These data indicated that CXCR2 downregulation increased chemotherapy sensitivity to doxorubicin and docetaxel in TNBC cells.
Discussion
Our study indicated that: (I) CXCR2 high expression was correlated with increased pathological grade, elevated N stage and advanced TNM stage in TNBC patients; (II) CXCR2 high expression was associated with shorter OS in TNBC patients; (III) CXCR2 downregulation increased the sensitivity to doxorubicin and docetaxel of TNBC cells.
Chemokines are not only revealed to support tumor growth but also implicated in tumor progression and the establishment of tumor cells at distant organ sites (10). CXCR2 is a G protein-coupled receptor that interacts with a wide range of chemokines, especially all angiogenic glutamic acid-leucine-arginine (ELR+) CXC chemokines that mediate angiogenic activity via interacting with CXCR2 (11,12,16). Recent studies have disclosed the role of CXCR2 in tumorigenesis and progression in vivo or in vitro (13,17-19). For example, CXCR2 knockdown inhibits tumor metastases and enhances antitumor effect of paclitaxel in mammary tumor model (13). Also, CXCR2 promotes cell proliferation via inhibiting p21 through protein kinase B (Akt)-Mdm2 signaling pathway in ovarian cancer (17). CXCR2 knockdown suppresses cell proliferation and chemotaxis, and induces cell apoptosis though extracellular regulated protein kinase (ERK) 1/2 pathways in esophageal cancer (18). Additionally, CXCR2 decreases cell apoptosis and enhances angiogenesis through multiple signaling pathways in ovarian cancer, such as mitogen-activated protein kinase and nuclear factor (NF)-κB pathways (19). These data suggested that CXCR2 might enhance cell proliferation, reduce cell apoptosis and decrease chemotherapy drug sensitivity through regulating several signaling pathways such as Akt-Mdm2, ERK1/2, mitogen-activated protein kinase and NF-κB pathways, thus, further contributing to the progression of these cancers.
Some literatures have disclosed that CXCR2 correlates with disease progression in several cancers (11,12). For example, a study shows that CXCR2 expression is positively correlated with TNM stage, lymph node metastases and depth of invasion in gastric adenocarcinoma (11). Another study discloses that CXCR2 expression positively associates with tumor size, Scarff, Bloom and Richardson (SBR) tumor grade, and lymph node metastases in breast cancer (12). These data reveal the positive correlation of CXCR2 expression with disease progression in some cancers, including breast cancer. Based on these indications, we speculated that CXCR2 might also take part in the disease progression of TNBC, which was the most deteriorated type of breast cancer. However, limited evidence about the role of CXCR2 in TNBC progression has been observed. In our study, we enrolled 158 TNBC patients to investigate the correlation of CXCR2 expression with tumor stages, and we observed that CXCR2 high expression was associated with increased pathological grade, raised N stage and elevated TNM stage in TNBC patients. Thid could be resulted from that: (I) CXCR2 might promote cell migration and invasion through regulating NF-κB and AKT pathways, which consequently facilitated tumor infiltration and tumor metastasis. Thus, CXCR2 high expression correlated with elevated N stage as well as TNM stage (17,20); (II) CXCR2 promoted stemness of cancer cells via mediating mTOR, β-catenin and hTERT activities, thereby resulted in the poorly differentiated tumors, which led to increased pathological grade in TNBC patients (21).
Emerging efforts have been paid to explore prognostic markers for predicting cancer outcomes, and CXCR2 has presented good predictive value for the prognosis of a few cancers (11,12,22). For instance, CXCR2 high expression is associated with shorter OS in gastric adenocarcinoma patients (11). As to breast cancer, a study shows that CXCR2 expression negatively correlates with OS and disease-free survival (12). Moreover, another study discloses that CXCR2 is an independent predictor for disease-free survival in breast cancer patients (22). However, limited evidence discloses the predictive value of CXCR2 for survival in TNBC patients. Our study showed that CXCR2 high expression independently predicted worse OS in TNBC patients, and the following reasons might explain: (I) CXCR2 might regulate several signaling pathways such as Akt-Mdm2 and ERK1/2 to promote cell migration and invasion as well as enhance stemness of cancer cells, which resulted in elevated TNM stage and higher pathological grade, thereby led to worse OS in TNBC patients (17,18); (II) CXCR2 decreased the sensitivity to doxorubicin and docetaxel in TNBC, which further impaired the treatment efficacy of neoadjuvant or adjuvant chemotherapy and resulted in reduced OS in TNBC patients.
To further investigate whether the predictive value of CXCR2 for TNBC prognosis was raised from its effect on regulating chemotherapy sensitivity, we performed CXCR2 downregulation in HCC1937 cells and evaluated the relative cell viability after treated by doxorubicin and docetaxel with different concentration, followed by the calculation of IC50 values of each drug. We observed that CXCR2 downregulation elevated the chemotherapy sensitivity of HCC1937 cells to doxorubicin and docetaxel, with decreased IC50 of doxorubicin and reduced IC50 of docetaxel. Our observation indicated that inhibiting CXCR2 increased the sensitivity to doxorubicin and docetaxel of TNBC cells. These results might be explained by: CXCR1/CXCR2 network mediated by NF-κB-dependent mechanism was previously observed to be closely implicated in chemotherapy resistance, thus targeting CXCR2 increased chemotherapy sensitivity to doxorubicin and docetaxel (23).
There are still some limitations in this study: (I) the sample size (N=158) was relatively small, which might cause relatively low statistical efficacy; (II) as a retrospective study, only formalin-fixed paraffin-embedded tissues were used for detecting CXCR2 expression; (III) although we evaluated the effect of CXCR2 downregulation on chemotherapy sensitivity to doxorubicin and docetaxel in TNBC cells, the underlying molecular mechanisms still needed further exploration.
In conclusion, CXCR2 high expression correlates with increased pathological grade, elevated N stage as well as raised TNM stage, and predicts shorter OS in TNBC patients, moreover, its downregulation enhances chemotherapy sensitivity to doxorubicin and docetaxel in TNBC cells. Therefore, CXCR2 has the potential to serve as a biomarker for assisting TNBC management, and targeting CXCR2 provides a novel strategy to circumvent the chemotherapy resistance.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of our hospital, and all patients or their family members provided written informed consents or verbal agreements with recording.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee A, Djamgoz MBA. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev 2018;62:110-22. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Jin K, Pandey NB, Popel AS. Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget 2017;8:60210-22. [Crossref] [PubMed]
- Kumar S, Wilkes DW, Samuel N, et al. DeltaNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Invest 2018;128:5095-109. [Crossref] [PubMed]
- Gluz O, Liedtke C, Gottschalk N, et al. Triple-negative breast cancer--current status and future directions. Ann Oncol 2009;20:1913-27. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017;7:1543-88. [Crossref] [PubMed]
- Liu Q, Li A, Tian Y, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev 2016;31:61-71. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Sharma B, Nannuru KC, Saxena S, et al. CXCR2: A Novel Mediator of Mammary Tumor Bone Metastasis. Int J Mol Sci 2019;20:1237. [Crossref] [PubMed]
- Yang SB, Han F, Wu JH, et al. Association between CXCR2 and IL-22BP expression indicate a poor outcome for gastric adenocarcinoma progression. Oncol Lett 2016;12:1477-84. [Crossref] [PubMed]
- Snoussi K, Mahfoudh W, Bouaouina N, et al. Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer 2010;10:283. [Crossref] [PubMed]
- Sharma B, Nawandar DM, Nannuru KC, et al. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol Cancer Ther 2013;12:799-808. [Crossref] [PubMed]
- Li RH, Huang WH, Wu JD, et al. EGFR expression is associated with cytoplasmic staining of CXCR4 and predicts poor prognosis in triple-negative breast carcinomas. Oncol Lett 2017;13:695-703. [Crossref] [PubMed]
- Corkery B, Crown J, Clynes M, et al. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol 2009;20:862-7. [Crossref] [PubMed]
- Ahuja SK, Lee JC, Murphy PM. CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B. Determinants of high affinity binding and receptor activation are distinct. J Biol Chem 1996;271:225-32. [Crossref] [PubMed]
- Ignacio RMC, Dong YL, Kabir SM, et al. CXCR2 is a negative regulator of p21 in p53-dependent and independent manner via Akt-mediated Mdm2 in ovarian cancer. Oncotarget 2018;9:9751-65. [Crossref] [PubMed]
- Wu K, Cui L, Yang Y, et al. Silencing of CXCR2 and CXCR7 protects against esophageal cancer. Am J Transl Res 2016;8:3398-408. [PubMed]
- Yang G, Rosen DG, Liu G, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res 2010;16:3875-86. [Crossref] [PubMed]
- Nannuru KC, Sharma B, Varney ML, et al. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog 2011;10:40. [Crossref] [PubMed]
- Jung JH, Kang KW, Kim J, et al. CXCR2 Inhibition in Human Pluripotent Stem Cells Induces Predominant Differentiation to Mesoderm and Endoderm Through Repression of mTOR, beta-Catenin, and hTERT Activities. Stem Cells Dev 2016;25:1006-19. [Crossref] [PubMed]
- Xu H, Lin F, Wang Z, et al. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett 2018;412:69-80. [Crossref] [PubMed]
- Wu S, Saxena S, Varney ML, et al. CXCR1/2 Chemokine Network Regulates Melanoma Resistance to Chemotherapies Mediated by NF-kappaB. Curr Mol Med 2017;17:436-49. [PubMed]


