Prevalence and genotypes of human papilloma virus infection in CIN3 in Beijing, China
Introduction
Before the occurrence of cervical cancer, a period of precancerous lesion, which is known as cervical intraepithelial neoplasia (CIN), is divided into three grades according to the proportion of mature and differentiated cells to the thickness of epithelial cells (1). CIN lesions can be subsided, constant, or progressing, most of the low grade CIN can be reduced or not progressed in a short time, but higher level CIN is more likely to develop for invasive cervical cancer. A larger epidemiological research found that cervical precancerous lesions and cervical cancer are associated with a variety of risk factors, among which HPV infection is the most important risk factor (2-4).
Human papillomavirus (HPV) infection is one of the most common female genital tract infections, which can be divided into high risk and low risk types according to their carcinogenic potential. Persistent infection of high risk HPV is not only a necessary condition for CIN and cervical invasive cancer, but also as a predictor of recurrence or residual in the patients with high level CIN (5,6). The distribution of HPV subtypes has regional differences and is affected by the degree of cervical lesions, however, in most researches, HPV16 has been found as the most widely distributed type in cervical and advanced precancerous lesions (7,8). In recent years, the HPV vaccine has been recognized as an important role in the prevention of HPV related precancerous lesions and cervical cancer (9). However, the current HPV vaccine does not contain all HPV subtypes, and the distribution of HPV subtypes is different in different regions. It is not clear whether the current HPV vaccine is effective in different regions. Therefore, this study was designed to analyze the distribution of HPV type, single and multiple infection in CIN3 patients, and provided data reference for the selection of HPV vaccine and the regionalization design of HPV vaccine.
Methods
Patients
The complete clinical data of the patients diagnosed with CIN3 by biopsy in Beijing Obstetrics and Gynecology Hospital, Capital Medical University from January 2012 to December 2017, were collected.
Inclusion criteria: (I) CIN3 was diagnosed by biopsy of the colposcopy, and the initial cervical cold knife coning (CKC) was first accepted; (II) the patient received HPV-23 test and at least one HPV type positive within 3 months before treatment; (III) no autoimmune disease or oral immune suppression. Exclusion criteria: (I) suspected early cervical invasive cancer or cervical cancer; (II) pathological non CIN3 patients after cervical conization; (III) HPV was negative or unclear before treatment; (IV) not regularly followed up; (V) pregnancy, lactation women; (VI) other malignant tumors. (VII) death or loss of visit to the patient.
A total of 679 patients were included in the final analysis. The written informed consents were obtained from all the patients. This study was approved by the ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University.
Sample collection and HPV detection
HPV samples were collected within one month before the operation, and the special cervical exfoliated cell harvester was used for sampling. By exposing the cervix through the vagina, wipe off the excessive secretion of the cervix with a cotton swab, and extend the cervical brush into the cervix, and gently rub the cervix brush to make it rotate for 4–5 rounds in a single direction and obtain a full amount of epithelial cell samples. Then the cervical brush head is placed in the sample tube containing the cell preservation solution (Shenzhen MIRACLEAN Technology Co., Ltd., China). The cervical brush handle is broken along the crease of the brush handle. The sample tube cover is tightened and marked. Samples were stored at 4°C and examined within 7 days.
The subtype of HPV was detected by DNA chip and PCR amplification using a nucleic acid typing test kit (5218-2011, China). 23 HPV genotypes, including 18 high risk types: HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 59, 66, MM4, and HPV6 43, 44 were detected. The measurement was performed according to the manufactory’s instruction.
Multiple HPV infection is defined as a single sample containing two or more than two kinds of HPV type.
Operation
All the patients were treated with cervical cold knife conization. Under general anesthesia, the patients were taken the bladder stone position and exposed to the cervix with a right angle vaginal speculum. The iodine staining test was used to determine and describe the atypical hyperplasia area of the cervix. The size and shape of the cone were designed according to the scope of the cervical lesions and the shape of the cervix. The rat tooth forceps pulled the anterior lip of the cervix to explore the depth and flexion of the uterine cavity. A circular incision was made at the surface of 5–10 mm cervix from the outside of the cervical canal, or outside the edge of the iodine non coloring area, with the tip of the cervix. The depth of the incision should be up to the cervical interstitium. The length of the incision should reach 2–2.5 cm, which includes the total length of the cervical canal. The cervical vertebrae were slanted to the cervix, and the cervix was completely removed by scissors. The conical specimens were resected at 12 points, with 10% formalin fixed for pathological examination.
Statistical analysis
SPSS17.0 software was used for statistical analysis, and chi square test was used for enumeration data. P<0.05 was statistically significant.
Results
HPV type distribution
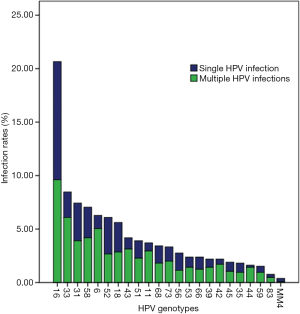
From January 2012 to December 2017, 679 patients with HPV (+) CIN3 were treated with primary conization. A total of 679 cases were detected, including 437 cases of single HPV subtype infection, 148 cases of HPV subtype infection, 3 HPV subtypes in 69 cases (Table 1). The average age of the patients was 22–68 years (39.08±8.216 years). The distribution and multiple infections of different HPV subtypes were shown in Figure 1 and Table 2. In high risk HPV genotypes, HPV16 has the highest single infection rate and multiple infection rates.
Table 1
| HPV genotypes | No. of cases |
|---|---|
| 1 type | 437 (64.36) |
| 2 types | 148 (21.80) |
| 3 types | 69 (10.16) |
| 4 types | 16 (2.36) |
| ≥5 types | 9 (1.33) |
| Total | 679 (100.0) |
Table 2
| HPV genotypes | Infections | ||
|---|---|---|---|
| Single (n, %) | Multiple (n, %) | Total (n, %) | |
| High-risk | |||
| 16 | 116 (53.46) | 101 (46.54) | 217 (100.0) |
| 18 | 29 (49.15) | 30 (50.85) | 59 (100.0) |
| 31 | 37 (47.44) | 41 (52.56) | 78 (100.0) |
| 33 | 25 (28.09) | 64 (71.91) | 89 (100.0) |
| 35 | 9 (47.37) | 10 (52.63) | 19 (100.0) |
| 39 | 8 (34.78) | 15 (65.22) | 23 (100.0) |
| 45 | 9 (45.00) | 11 (55.00) | 20 (100.0) |
| 51 | 17 (41.46) | 24 (58.54) | 41 (100.0) |
| 52 | 36 (56.25) | 28 (43.75) | 64 (100.0) |
| 53 | 10 (40.00) | 15 (60.00) | 25 (100.0) |
| 56 | 17 (58.62) | 12 (41.38) | 29 (100.0) |
| 58 | 30 (40.54) | 44 (59.46) | 74 (100.0) |
| 59 | 6 (37.50) | 10 (62.50) | 16 (100.0) |
| 66 | 12 (48.00) | 13 (52.00) | 25 (100.0) |
| 68 | 17 (47.22) | 19 (52.78) | 36 (100.0) |
| 73 | 14 (40.00) | 21 (60.00) | 35 (100.0) |
| 83 | 3 (37.50) | 5 (62.50) | 8 (100.0) |
| MM4 | 3 (75.00) | 1 (25.00) | 4 (100.0) |
| Low-risk | |||
| 6 | 13 (19.70) | 53 (80.30) | 66 (100.0) |
| 11 | 8 (20.51) | 31 (79.49) | 39 (100.0) |
| 42 | 5 (21.74) | 18 (78.26) | 23 (100.0) |
| 43 | 11 (25.00) | 33 (75.00) | 44 (100.0) |
| 44 | 2 (11.76) | 15 (88.24) | 17 (100.0) |
Single and multiple HPV infection in different time periods
Among the 679 CIN3 patients enrolled, the single subtype HPV infection accounted for 64.36% (437/679) and the multiple HPV infection accounted for 35.64% (242/679). It was divided into two groups, 2012–2014 year group and 2015–2017 year group. In 2012–2014 year group, the single subtype HPV infection rate was 69.92% (179/256), while multiple HPV infection rate was 30.08% (77/256). In 2015–2017 year group, the single and multiple HPV infection rate was 60.99% (258/423), 39.01% (165/423) respectively (Table 3). The proportion of multiple HPV infection between two groups was significant different (39.01% vs. 30.08%, P=0.019).
Table 3
| Calendar years | No. of conizations | Single HPV positive (n%) | Multiple HPV positive (n%) |
|---|---|---|---|
| 2012–2014 | 256 | 179 (69.92) | 77 (30.08) |
| 2015–2017 | 423 | 258 (60.99) | 165 (39.01) |
*, χ2=5.543, P=0.019.
Single and multiple HPV infection in different age groups
The ratio of multiple HPV infections in the 20–29 years old group, the 30–39 years old group, the 40–49 years old group and the 50 years old group was 38.27%, 41.73%, 32.94%, 16.92%, respectively (Table 4). The number of CIN3 patients aged 30–39 years was the largest, and the proportion of multiple HPV infection in this age group was the largest, while CIN3 patients aged 50 and above were the least, and the multiple infection rate in this age group was the least.
Table 4
| Age groups | No. of conizations | Single HPV positive (n%) | Multiple HPV positive (n%) |
|---|---|---|---|
| 20–29 | 81 | 50 (61.73) | 31 (38.27) |
| 30–39 | 278 | 162 (58.27) | 116 (41.73) |
| 40–49 | 255 | 171 (67.06) | 84 (32.94) |
| ≥50 | 65 | 54 (83.08) | 11 (16.92) |
| Total | 679 | 437 (64.36) | 242 (35.64) |
Discussion
The distribution of HPV subtype and single and multiple infections in CIN3 patients were investigated. The results showed that in 679 patients with HPV (+) CIN3, single subtype HPV infection accounted for 64.36% (437/679), and multiple HPV infection accounted for 35.64% (242/679). Beca et al. (10) showed that 36.93% of 176 HSIL patients had multiple HPV infection. In addition, the proportion of multiple HPV infection in some other high grade cervical lesions was about 40% compared with the HPV study (11,12). However, the proportion of multiple HPV infections is very different in many large sample studies (13,14). It might be caused by the differences in research design, research population, HPV detection techniques and so on.
Among the 242 cases of multiple HPV infection, 2 HPV subtypes accounted for 61.16% (148/242), and 3 HPV subtypes accounted for only 28.51% (69/242). The more HPV subtypes were infected in the same patient, the smaller the proportion. Type HPV16 is the most widely distributed subtype of multiple HPV infections, accounting for 41.74% (101/242), but the proportion of HPV16 combined with other subtypes is not the largest, which is 46.54% (101/217). Compared with the proportion of the multiple subtypes of other subtypes, the HPV16 may be less likely to be infected with other subtypes. However, it is not clear whether cross immune or synergistic effects exist among different HPV subtypes. Dickson et al. (13) examined the HPV distribution of cervical cytology and the correlation of multiple HPV infections in 309,000 cases. The results showed that HPV52, 53, 81, and 83 were more likely to be infected with other HPV, and HPV16, 58, and 66 were less likely to be infected with other HPV subtypes. Moreover, HPV in group-alpha 9 was not easy to co infection with other HPV groups. It is concluded that there may be competition and synergy between different HPV subtypes. Moreover, Trottier et al. (15) demonstrated that HPV in group -9 can affect the infection of HPV in other groups. However, Tota et al. (16) evaluated the possibility of infection with other subtypes of HPV in patients vaccinated with HPV4 (including HPV6, 11, 16, 18). The results showed that patients who were vaccinated with HPV4 were still likely to infect other HPV subtypes. It is suggested that there is no competition relationship between the 4 HPV subtypes and other HPV subtypes. Further researches are needed on whether there is competition or synergy between different HPV subtypes.
Among all the detected HPV types, HPV16, 33, 31, 58, 6, 52, 18, 43, 51, 11 were the most widely distributed HPV subtypes. In one research with large number samples, HPV16 was found as the most widely distributed subtype of all levels of CIN (8,17,18). But in other two researches, HPV52 was shown as the most widely distributed type, and HPV16 ranks second (19,20). The distribution of HPV can vary from region to group. Interestingly, in this study, the proportion of low risk HPV6 and 11 was 6.28% and 3.9% respectively. The proportion of multiple infections of these two types of HPV accounted for 80.3% and 79.4% respectively. These two subtypes may be more likely to merge with other high-risk types, while high-risk types are the main cause of CIN3.
HPV multiple infection has attracted much attention in recent years. Some studies have found that HPV multiple infection rate has been increasing in recent years (21,22). In this study, the difference of multiple HPV infection rate of 2015−2017 is higher than 2012–2014 year group, which suggested that multiple HPV infection is also increasing in CIN3. A longitudinal evaluation of HPV infection and HPV genotype distribution using four distinct mathematical approaches among 10,656 women enrolled in the placebo arms of three phase III clinical trials of the qHPV vaccine has been performed (23). Approximately 85% or more of CIN3/AIS, >70% CIN2, and approximately 50% of CIN1 lesions worldwide are attributed to HPV6/11/16/18/31/33/45/52/58 (23). From that result, it can be concluded that if 9-valent HPV vaccination programs are effectively implemented, the majority of CIN2 and CIN3 lesions worldwide could be prevented, in addition to approximately one-half of CIN1, which was partly in accordance with our results.
It is well known that cervical lesions are a process from normal to low grade lesions and further to the progression of high-grade lesions. High-risk HPV plays a major role in the development of cervical lesions. As the severity of cervical lesions progresses, the proportion of the more carcinogenic HPV is increasing. Moreover, the distribution of high-risk HPV is different in different regions.
As noted, both cervical cytology and high-risk HPV DNA testing can detect cervical cancer and its precursors, but each also detect abnormalities that will not go on to become cancer (24). Although annual screening with cytology alone has saved many lives, it has also been shown to increase the number of unnecessary procedures and treatments (24). HPV testing has been recommended as the primary screening tool in women 25 years and older to assess risk of cervical cancer in western countries (25). However, in China, the implementation of HPV screening is difficult. In this way, the HPV vaccine seems to have higher application value.
Conclusions
In conclusion, this study analyzed the situation and trend of single and multiple HPV infection in CIN3 patients in Beijing. The first ten HPV subtypes of the highest rate of infection in CIN3 patients were 16, 33, 31, 58, 6, 52, 18, 43, 51, 11, all of which were included in the HPV nine vaccine except HPV43 and 51. The proportion of multiple infections in HPV was increased. In CIN3, multiple infections were more common in patients aged 30–39 years. This study provides basic data for the regionalization of HPV vaccine in Beijing.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.59). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University. The written informed consents were obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wheeler CM. The natural history of cervical human papillomavirus infections and cervical cancer: gaps in knowledge and future horizons. Obstet Gynecol Clin North Am 2013;40:165-76. [Crossref] [PubMed]
- Ostör AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993;12:186-92. [Crossref] [PubMed]
- Holowaty P, Miller AB, Rohan T, et al. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 1999;91:252-8. [Crossref] [PubMed]
- Ward KK, Shah NR, Saenz CC, et al. Changing demographics of cervical cancer in the United States (1973-2008). Gynecol Oncol 2012;126:330-3. [Crossref] [PubMed]
- Acladious NN, Sutton C, Mandal D, et al. Persistent human papillomavirus infection and smoking increase risk of failure of treatment of cervical intraepithelial neoplasia (CIN). Int J Cancer 2002;98:435-9. [Crossref] [PubMed]
- Serati M, Siesto G, Carollo S, et al. Risk factors for cervical intraepithelial neoplasia recurrence after conization: a 10-year study. Eur J Obstet Gynecol Reprod Biol 2012;165:86-90. [Crossref] [PubMed]
- Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007;121:621-32. [Crossref] [PubMed]
- Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 2013;132:198-207. [Crossref] [PubMed]
- Hariri S, Bennett NM, Niccolai LM, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008-2012. Vaccine 2015;33:1608-13. [Crossref] [PubMed]
- Beca F, Pinheiro J, Rios E, et al. Genotypes and prevalence of HPV single and multiple concurrent infections in women with HSIL. Diagn Cytopathol 2014;42:919-23. [Crossref] [PubMed]
- Schmitt M, Depuydt C, Benoy I, et al. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol 2013;51:1458-64. [Crossref] [PubMed]
- Pista A, Oliveira A, Verdasca N, et al. Single and multiple human papillomavirus infections in cervical abnormalities in Portuguese women. Clin Microbiol Infect 2011;17:941-6. [Crossref] [PubMed]
- Dickson EL, Vogel RI, Bliss RL, et al. Multiple-type human papillomavirus (HPV) infections: a cross-sectional analysis of the prevalence of specific types in 309,000 women referred for HPV testing at the time of cervical cytology. Int J Gynecol Cancer 2013;23:1295-302. [Crossref] [PubMed]
- Carozzi F, Ronco G, Gillio-Tos A, et al. Concurrent infections with multiple human papillomavirus (HPV) types in the New Technologies for Cervical Cancer (NTCC) screening study. Eur J Cancer 2012;48:1633-7. [Crossref] [PubMed]
- Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2006;15:1274-80. [Crossref] [PubMed]
- Tota JE, Ramanakumar AV, Villa LL, et al. Cervical Infection With Vaccine-Associated Human Papillomavirus (HPV) Genotypes as a Predictor of Acquisition and Clearance of Other HPV Infections. J Infect Dis 2016;214:676-84. [Crossref] [PubMed]
- Chen X, Wallin KL, Duan M, et al. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol 2015;87:1966-72. [Crossref] [PubMed]
- Li K, Yin R, Li Q, et al. Analysis of HPV distribution in patients with cervical precancerous lesions in Western China. Medicine (Baltimore) 2017;96:e7304. [Crossref] [PubMed]
- Wang H, Cheng X, Ye J, et al. Distribution of human papilloma virus genotype prevalence in invasive cervical carcinomas and precancerous lesions in the Yangtze River Delta area, China. BMC Cancer 2018;18:487. [Crossref] [PubMed]
- Lu S, Cong X, Li M, et al. Distribution of high-risk human papillomavirus genotypes in HPV-infected women in Beijing, China. J Med Virol 2015;87:504-7. [Crossref] [PubMed]
- Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011;128:927-35. [Crossref] [PubMed]
- Sohrabi A, Hajia M, Jamali F, et al. Is incidence of multiple HPV genotypes rising in genital infections? J Infect Public Health 2017;10:730-3. [Crossref] [PubMed]
- Joura EA, Ault KA, Bosch FX, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev 2014;23:1997-2008. [Crossref] [PubMed]
- Jun J. Cancer/health communication and breast/cervical cancer screening among Asian Americans and five Asian ethnic groups. Ethn Health 2018; [Epub ahead of print].
- Castle PE, Stoler MH, Wright TC, et al. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011;12:880-90. [Crossref] [PubMed]