
Long non-coding RNA MEG3 functions as a competing endogenous RNA of miR-93 to regulate bladder cancer progression via PI3K/AKT/mTOR pathway
Introduction
Bladder cancer (BC) is emerged as a highly prevalent malignancy associated with high morbidity and mortality and its incidence has increased over the last decades (1,2). At present, various therapies including surgery, chemotherapy and radiation treatment have been widely applied in the therapy of BC (1). However, the advances in BC treatment have been greatly restricted in the past decades due to the lack of understanding of its pathogenic mechanism. Thus, it is urgent to elaborate the pathogenic mechanism of BC. Recently, long noncoding RNAs (lncRNAs) have emerged as the crucial regulators in the tumorigenesis and metastasis (3), which could function as the tumor suppressor or oncogene in the tumorigenesis (4).
Maternally expressed gene 3 (MEG3) as a lncRNA was reported to serve as a tumor suppressor in human cancer cell lines and tissues (5,6). MEG3 is expressed in various normal human tissues, however its expression is down-regulated in the tumors and tumor cell lines (7). Previous study has demonstrated that MEG3 overexpression could promote apoptosis and inhibit proliferation (8). Particularly, the MEG3 knockdown was proved to regulate the proliferation and autophagy in BC cells (9). Therefore, we speculated that MEG3 might have inhibitory effects in the development and BC progression and can act as potential drug in anti-tumor therapy. However, the underlying mechanism is still unclear.
It is well-accepted that lncRNAs sever as competing endogenous RNAs (ceRNAs) or sponges of microRNA (miRNAs) to indirectly regulate mRNAs expression by competing for binding to shared miRNAs (10). For example, Huang et al. indicated that MEG3 impairs BC invasion by binding with miR-27a (11). Importantly, Zhang et al. previously reported that MEG3 binds with miR-93 to inactivate PI3K/AKT pathway and thereby inhibits of glioma cell growth (12). Mammalian target of rapamycin (mTOR) is the downstream of PI3K/AKT pathway, which has effects in regulating differentiation, proliferation and controls whether a cell undergoes programmed cell apoptosis or autophagy (13). Thus, we speculated that MEG3 may also affect the BC cell proliferation by targeting miR-93-5p and inactivating PI3K/AKT/mTOR pathway. Therefore, we aimed to demonstrate effects of MEG3 on BC cell proliferation and elaborate whether MEG3 regulate the BC progression by miR-93-5p and PI3K/AKT/mTOR pathway.
Methods
Human samples
Twenty pairs of surgical samples (BC tissues and normal tissues) were originally obtained from patients underwent surgical resection in Peking Union Medical College Hospital (Beijing, China) from 2017 to 2018. Patients who not received chemotherapy and radiotherapy before surgical resection were eligible for enrollment. Samples were frozen in liquid nitrogen and stored in the −80 °C refrigerator until used. This study was approved by Ethics Committee of Peking Union Medical College Hospital (approval number: SYXK(Beijing)2018-0027). Informed consents were obtained from each patient before operation.
Cell lines and culture
Four BC cell lines (T24, UMUC3, 5637 and BIU87) and normal bladder epithelial SV-HUC-1 cell lines were originally obtained from Shanghai Institutes for Biological Sciences (Shanghai, China). Cells were grown in RPMI-1640 medium supplementing with 10% heat-in-activated fetal bovine serum (Gibco, USA), 100 µg/mL streptomycin and 100 U/mL penicillin. Subsequently, cell lines were maintained in an incubator at 37 °C with 5% CO2.
RNA extraction and qRT-PCR
Trizol reagent (Takara, Japan) was used to extract total RNA from cell lines and tissues samples. Reverse Transcriptase Kit was used to reversely transcribe RNA into cDNA (Takara, China). Then, mRNA expression was detected by SYBR Premix Ex Taq (Takara, China) on the ABI 7300 system (Applied Biosystems, USA). The relative expression of mRNAs was evaluated by 2−ΔΔCt method, using GAPDH and small nuclear RNA U6 as the internal control for miR-93-5p and MEG3, respectively. PCR primer sequences were showed in Table 1.
Table 1
| Gene | Forward | Reverse |
|---|---|---|
| miR-93-5p | 5'-CAAAGTGCTGTTCGTGCAGGTAG-3' | 5'-GGATCCGACGGCTGGGTCTTCTCAGA-3' |
| U6 | 5'-TGCGGGTGCTCGCTTCGGCAGC-3' | 5'-GGATCCGACGGCTGGGTCTTCTCAGA-3' |
| GAPDH | 5'-CTGGGCTACACTGAGCACC-3' | 5'-AAGTGG TCGTTGAGGGCAATG-3' |
| MEG3 | 5'-CTGCCCATCTACACCTCACG-3' | 5'-CTCTCCGCCGTCTGCGCTAGGGGCT-3' |
Cell transfection
Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Canada). Recombinant lentiviral vector carrying lncRNA MEG3 or short hairpin RNAs (shRNA)-MEG3 were constructed to induce MEG3 up-regulation or down-regulation as previously described (12). The miR-93-5p mimics were obtained from Genepharma (Shanghai, China) to induce miR-93-5p up-regulation. BIU87 cells were transfected with lentiviral vector lncRNA MEG3 and/or miR-93-5p mimics, respectively. Untransfected cells were used as a blank control.
Luciferase reporter assay
The MEG3 3’-UTR (WT) of the Renilla luciferase gene was cloned into psiCHECK2 dual-luciferase vector (Promega, USA). After 24 hours, cells (1×105 cells/well) were co-transfected with WT or MUT luciferase constructs (0.4 ng/µL) and miR-93-5p mimics by Lipofectamine 2000 (Invitrogen, USA). After 72 hours transfection, Renilla and Firefly luminescence were analyzed.
Cell viability assay and apoptosis assay
Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Japan) was used to evaluate the cell viability. The miR-93-5p mimics or/and LncRNA-MEG3, or their negative control respectively transfected BIU87 cells. After 48 hours transfection, cells at a density of 5×103 were seeded into 96-well plates and then incubated for 1–4 days, respectively. After incubation, CCK-8 solution were added and then incubated for another 2 hours at 37 °C. The absorbance was measured at 450 nm.
Cell apoptosis was measured by the flow cytometry assay. After transfection and incubation, cells were double-labeled with Annexin V-fluorescein isothiocyanate and propidium iodide apoptosis detection kits (eBioscience, USA). Then, cells were analyzed immediately in a BD FACSCalibur flow cytometer (BD Biosciences, USA).
Western blot assays
Primary antibodies to Ki67, PCNA, Caspase-3, cleaved Caspase-3, Caspase-9, cleaved Caspase-9, LC3II, LC3I, Beclin1, p53, Akt, p-Akt, mTOR, p-mTOR, Ulk, p-Ulk and GADPH (endogenous control) were used in the western blot assay. All primary antibodies were purchased from Abcam Cambridge, UK.
Total protein lysates were isolated by RIPA lysis buffer. Firstly, isolated protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). PVDF membrane was then blocked by an incubation in phosphate-buffered saline (PBS) for 2 h at 37 °C. Subsequently, PVDF membranes were incubated with specific primary antibodies separately at 4 °C overnight. GAPDH was used as internal control. PVDF membrane were stained with the corresponding HRP-conjugated secondary antibodies (Abcam, UK), followed by visualized with enhanced chemiluminescence (ECL) system (GE Health-care, USA), and analyzed using Chemi-Doc XRS (Bio-rad, USA).
5-ethynyl-2’-deoxyuridine (EdU) staining
Cells were treated with EdU (50 µmol/L) and then fixed in 4% paraformaldehyde for 10 min at room temperature. Cells were permeabilized with TritonX-100 at 37 °C for 30 min after being washed PBS twice. Cells were then reacted with Apollo reaction cocktail (1×, 100 µL) for 30 min, and stained with Hoechst 33342 (5 µg/mL, 100 µL) for 30 min. Finally, cells were visualized under a fluorescent microscope.
Immunofluorescence
Cells were cultured to 70% confluence, washed twice, and fixed with 4% paraformaldehyde at room temperature for 15 min. After blocking with 1% bovine serum albumin, cells were incubated with primary antibody against LC3 (Abcam, ab58610). Then, cells were washed with PBS, and stained with secondary antibody to conjugated to HRP at room temperature for 50 min. Cells were visualized under a Leica TCS SP II confocal laser scanning microscope (Leica, USA).
Xeno-graft mouse model
Forty male specific pathogen-free athymic nude mice (weighing 20–25 g, aged 6–8 weeks) were obtained from Shanghai National Center for Laboratory Animals (Shanghai, China). Mice were housed under specific pathogen-free conditions at humidity of 25±2 °C/60%±5%, 12 hours light/dark cycle, free access to food and water. Animal experiment was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Thirty SPF nude mice were subcutaneously injected with 1×107 BIU87 cells transfected with lncRNA-MEG3 or/and miR-93-5p mimics randomly. Another ten mice were used as blank control. Then, the volume of tumor nodules was detected using external caliper every 6 days. The following formula was used to calculate tumor volumes: Volume = (width + length)/2 × width × length × 0.5236. After 30 days, all mice were sacrificed for immunohistochemistry assay. Tumor tissue was sectioned and fixed in 10% formalin for 48 hours, followed by paraffin embedded. Then, the tissue section was incubated with primary antibody anti Ki-67 (Dako, 1:50 dilution) at 4 °C for 12 hours and anti-Caspase3 (Dako, 1:50 dilution). Secondary antibody streptavidin-HRP-conjugated (Santa Cruz, CA, USA) was stained for 1 hour subsequently.
Statistical analysis
All statistical analyses were analyzed by SPSS 16.0 (IBM SPSS Inc., USA). All experiments were repeated in at least triplicate. Data are presented as mean ± standard deviation (SD). Comparison analysis was performed by Student’s t-test and one-way analysis of variance (ANOVA) when appropriate. The P value less than 0.05 was considered as statistically significant.
Results
Differential expression of MEG3 and miR-93-5p in BC cells and tissues
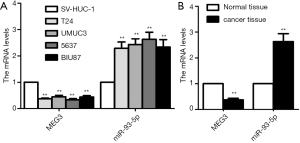
To evaluate the role of MEG3 and miR-93-5p in BC progression, qRT-PCR was used to measure the expression in BC cell lines and tissues respectively. MEG3 was down-regulated in all BC cells (T24, UMUC3, 5637 and BIU87) compared to SV-HUC-1 cell (all P<0.01, Figure 1A). Conversely, miR-93-5p was up-regulated in BC cell lines compared with SV-HUC-1 cell (all P<0.01, Figure 1A). For the expression in BC cells, MEG3 expression level was highest while miR-93-5p level was lowest in BIU87 cells compared to other three cell lines (Figure 1A). Consistently, MEG3 was significantly down-regulated and miR-93-5p was overexpressed in BC tissues compared to normal tissues (n=20 in each group, Figure 1B). Overall, these results suggested the MEG3 and miR-93-5p may participate in BC progression. In present study, BIU87 cell line was used to perform the subsequent experiments since it had the highest MEG3 and lowest miR-93-5p expression level.
MiR-93-5p is a direct target of MEG3
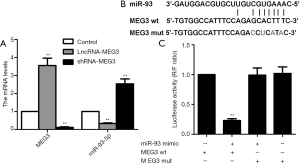
Subsequently, MEG3 was knocked down or overexpressed by expressing shRNA-MEG3 or LncR-MEG3 in BIU87 cell line to confirm the function of MEG3, respectively. As expected, the mRNA expression of MEG3 was significantly up-regulated or down-regulated compared to the control (Figure 2A). Furthermore, the miR-93-5p expression was significantly reduced by MEG3 overexpression, while enhanced by MEG3 knock-down (Figure 2A), indicating that miR-93-5p expression may be regulated by MEG3. Thereby, to verify the regulatory mechanism of MEG3 and miR-93-5p, a bioinformatics analysis was performed in the RegRNA 2.0 databases (http://regrna2.mbc.nctu.edu.tw/) for its potential target sites. As shown in Figure 2B, the 3’UTR of miR-93-5p harbors a consequential binding site for MEG3, indicating that miR-93-5p may be the potential regulatory target of MEG3. Then luciferase reporter assay further demonstrated that relative luciferase activity was inhibited in BIU87 cell line co-transfected with miR-93-5p and MEG3 (Figure 2C). Overall, these results suggested that miR-93-5p is a direct target of MEG3, and negatively regulated by MEG3.
MEG3 overexpression inhibited the tumorigenesis of BC cells by targeting miR-93-5p
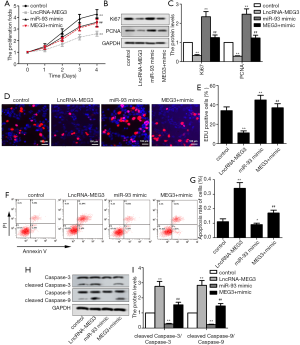
To investigate the potential molecular mechanism of MEG3 and miR-93-5p in BC progression, LncRNA-MEG3 and/or miR-93-5p mimics were transfected into BIU87 cell line. CKK-8 assay demonstrated that the cell proliferation was significantly inhibited by MEG3 up-regulation, while the miR-93-5p overexpression reversed the MEG3 overexpression-mediated suppression on cell proliferation (Figure 3A). Next, the expression of proliferation-related proteins including Ki67 and proliferating cell nuclear antigen (PCNA) were measured. As shown in Figure 3B and C, MEG3 overexpression suppressed the expression levels of Ki67 and PCNA, while co-transfection with LncRNA-MEG3 and miR-93-5p mimics significantly enhanced the MEG3 overexpression-mediated suppression on protein expression. EdU staining was performed to further detect the cell proliferation. Consistently, MEG3 overexpression significantly decreased the rate of EdU positive cells, while co-transfection with LncRNA-MEG3 and miR-93-5p mimics evidently abrogated this effect (Figure 3D,E). Conversely, MEG3 overexpression significantly promoted cell apoptosis, while co-transfection with LncRNA-MEG3 and miR-93-5p mimics abrogated this effect (Figure 3F,G). Meanwhile, western blot assay revealed that enhanced expression of MEG3 significantly promoted the expression of pro-apoptotic proteins (cleaved caspase-3 and caspase-9), while miR-93-5p mimics reversed this effect (Figure 3H,I). Overall, these results suggested that MEG3 may function as a tumor suppressor gene in BC, and miR-93-5p function as an oncogene. Besides, MEG3 overexpression inhibited the tumorigenesis of BC cell lines by targeting miR-93-5p.
MEG3 overexpression inhibited autophagy and the activation of PI3K/AKT/mTOR pathway by targeting miR-93-5p
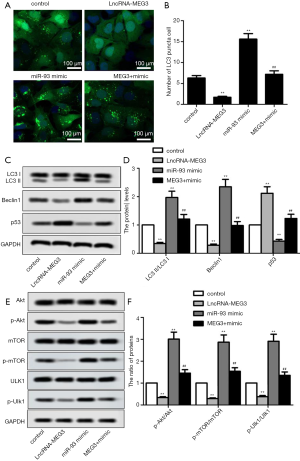
To explore whether MEG3 could regulate cell autophagy and PI3K/AKT/mTOR pathway by targeting miR-93-5p in BC cells, we measured the expression of autophagy-related proteins and proteins in PI3K/AKT/mTOR pathway in transfected BIU87 cells. Immunofluorescence presented a significant reduction in the number of LC3 punctated cells after transfection with LncRNA-MEG3, but miR-93-5p mimics completely reversed the result (Figure 4A,B). With regard to the expression of autophagy-related proteins, MEG3 overexpression significantly suppressed the autophagy-related proteins (LC3II/LC3I, Beclin1 and P53), while miR-93-5p mimics reversed the result (Figure 4C,D). Similarly, MEG3 overexpression decreased the rate of p-Akt/Akt, p-mTOR/mTOR and p-Ulk/Ulk, which was reversed by miR-93-5p up-regulation (Figure 4E,F). Overall, these results indicated MEG3 overexpression could inhibit autophagy and the activation of PI3K/AKT/mTOR pathway by targeting miR-93-5p in BC cell lines.
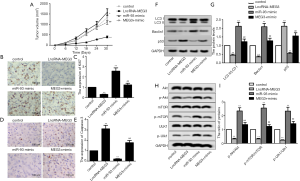
MEG3 overexpression inhibited tumorigenesis in vivo by targeting the miR-93-5p-mediated PI3K/AKT/mTOR pathway
To provide in vivo evidence for the regulatory mechanism of MEG3, xeno-graft mouse model was constructed to confirm the relationship between MEG3 and miR-93-5p. As shown in Figure 5A, the tumor volume was significantly reduced after MEG3 overexpression, while the overexpression of miR-93-5p reversed the MEG3 overexpression-mediated suppression on tumor volume. IHC staining using Ki67 antibody showed that MEG3 overexpression suppressed the expression levels of Ki67, while co-transfection with LncRNA-MEG3 and miR-93-5p mimics significantly reversed this result (Figure 5B,C). Inversely, MEG3 overexpression enhanced the caspase-3 expression, which was also reversed by miR-93-5p mimics (Figure 5D,E). Finally, we explored the influence of miR-93-5p on autophagy-related proteins and PI3K/AKT/mTOR pathway in vivo. Similarly with the in vitro experiments, MEG3 overexpression significantly decreased the rate of LC3II/LC3I, p-Akt/Akt, p-mTOR/mTOR and p-Ulk/Ulk, and also reduced the expression levels of Beclin1 and P53. However, miR-93-5p mimics completely reversed these results (Figure 5F,G,H,I). Together, these results indicated that MEG3 overexpression inhibited tumorigenesis in vivo by targeting miR-93-5p-mediated PI3K/AKT/mTOR pathway.
Discussion
To our knowledge, this is the first report focused on the regulatory mechanism of lncRNA MEG3 and miR-93 in BC progression. The present study demonstrated that lncRNA MEG3 functioned as a ceRNA of miR-93 to suppress autophagy and induce cell apoptosis by inactivating PI3K/AKT/mTOR pathway in the progression of BC.
We verified MEG3 was down-regulated in BC cell lines and tissues while miR-93-5p was up-regulated, indicating that lncRNA MEG3 and miR-93-5p may be involved in the BC progression. Previously, many studies have reported the down-regulation of lncRNA MEG3 and up-regulation of miRNAs in BC cell lines and tissues (14,15). Similarly, lncRNA MEG3 was decreased in many other tumors, such as esophageal squamous cell cancer (16), gastric cancer (7), lung cancer (17), and prostate cancer (18). These above evidences showed that lncRNA MEG3 may be an important regulatory factor of the tumorigenesis in various type of tumor.
lncRNAs acts as a sponge, could sequester miRNAs and indirectly regulates mRNA expression (19). lncRNA MEG3 previously was proved to have negative association with miR-494 and miR-27a in BC cell lines (14,20). Thus, we explored the relationship between lncRNA MEG3 and miR-93-5p. As expect, lncRNA MEG3 negatively regulated the expression of miR-93-5p. Luciferase reporter assay further demonstrated that MiR-93-5p is a direct target of MEG3.
To confirm the regulatory mechanism of lncRNA MEG3 and miR-93-5p in BC progression, BC cells and xeno-graft mouse models with abnormal lncRNA MEG3 and miR-93-5p expression were constructed. Either in vitro or in vivo, lncRNA MEG3 overexpression was showed to inhibit BC cell proliferation by decreasing the expression of proliferation markers (Ki67 and PCNA) and promote cell apoptosis by inhibiting pro-apoptotic proteins (cleaved caspase-3 and caspase-9), while miR-93-5p overexpression showed totally opposite results and promoted tumorigenesis. These results indicated that lncRNA MEG3 may function as a tumor suppressor gene while miR-93-5p as an oncogene. Multiple previous evidences demonstrated that overexpression of lncRNA MEG3 could suppress cell proliferation, migration and invasion (11,14,15), which were concordant with our present finding. More importantly, we observed that up-regulation of miR-93-5p completely reversed the MEG3 overexpression mediated-suppression on tumorigenesis in BC cell lines and xeno-graft mouse models. These results further confirmed that lncRNA MEG3 regulated the BC progression by targeting miR-93-5p. lncRNAs acts as ceRNAs, which could affect the post-transcriptional regulation by competing with targeting miRNAs and interfering with miRNA pathways (21,22). lncRNA MEG3 as a ceRNA of miR-19a, miR-181 and miRNA-664, was respectively demonstrated to regulate the carcinogenesis in glioma (23), gastric cancer (7), hepatocellular cancer (24). Notably, lncRNA MEG3 was also reported to sponge miR-494, thereby regulating the BC progression (14). Thus, we concluded that lncRNA MEG3 could function as a ceRNA of miR-93-5p to regulate the BC progression.
The miRNAs regulate the tumor growth through regulation of PI3K/AKT/mTOR pathway in various tumors (25). Among them, miR-93-5p was proved to promote metastasis and tumorigenesis of gliomas and non-small cell lung cancer by activating PI3K/AKT pathway (12,26,27). The study showed overexpression of lncRNA MEG3 decreased the rate of p-Akt/Akt, p-mTOR/mTOR and p-Ulk/Ulk, while miR-93-5p up-regulation reversed this effect. These results indicated that lncRNA MEG3 can regulate the PI3K/AKT/mTOR pathway through sponging miR-93-5p. Additionally, we observed that overexpression of lncRNA MEG3 could inhibit the autophagy-related proteins expression, which also be reversed by miR-93-5p up-regulation. This demonstrated that up-regulation of lncRNA MEG3 can also inhibit the autophagy, thereby resulting the increase of cell apoptosis in BC. This finding was consistent with Ying et al. who reported down-regulation of MEG3 increased cell proliferation and activated autophagy of BC (9). In addition, in vivo assays of xeno-graft mouse model also validated that lncRNA MEG3 could regulate autophagy and PI3K/AKT/mTOR pathway through sponging miR-93-5p in the tumorigenesis of BC.
Conclusions
Taken together, the present study demonstrated that lncRNA MEG3 functioned as a ceRNA of miR-93 to suppress autophagy and induce cell apoptosis by inactivating PI3K/AKT/mTOR pathway in BC progression. The present research provided a new perspective to understanding the pathogenic mechanism of BC. The inhibition effect of MEG3 on BC progression indicated that it may be a reliable diagnostic marker and effective therapeutic target for BC.
Acknowledgments
Funding: None.
Footnote
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.70). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of Peking Union Medical College Hospital (approval number: SYXK(Beijing)2018-0027). Informed consents were obtained from each patient before operation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016;388:2796-810. [Crossref] [PubMed]
- Gislefoss RE, Stenehjem JS, Hektoen HH, et al. Vitamin D, obesity and leptin in relation to bladder cancer incidence and survival: prospective protocol study. BMJ Open 2018;8:e019309. [Crossref] [PubMed]
- Lv M, Zhong Z, Huang M, et al. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res 2017;1864:1887-99.
- Liu B, Sun L, Liu Q, et al. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 2015;27:370-81. [Crossref] [PubMed]
- Yin DD, Liu ZJ, Zhang E, et al. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol 2015;36:4851-9. [Crossref] [PubMed]
- Ghafouri-Fard S, Taheri M. Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed Pharmacother 2019;118:109129. [Crossref] [PubMed]
- Peng W, Si S, Zhang Q, et al. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res 2015;34:79. [Crossref] [PubMed]
- Zhang X, Wu N, Wang J, et al. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J Cell Mol Med 2019;23:6708-19. [Crossref] [PubMed]
- Ying L, Huang Y, Chen H, et al. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol Biosyst 2013;9:407-11. [Crossref] [PubMed]
- Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014;505:344-52. [Crossref] [PubMed]
- Huang C, Liao X, Jin H, et al. MEG3 as a competing endogenous RNA binds with miR-27a to promote PHLPP2 protein translation, and consequently impairs bladder cancer invasion. Cancer Res 2017;77:1898.
- Zhang L, Liang X, Li Y. Long non-coding RNA MEG3 inhibits cell growth of gliomas by targeting miR-93 and inactivating PI3K/AKT pathway. Oncol Rep 2017;38:2408-16. [Crossref] [PubMed]
- Jacinto E, Werlen G. Mammalian Target of Rapamycin (mTOR). Compendium of Inflammatory Diseases 2016:874-92.
- Shan G, Tang T, Xia Y, et al. MEG3 interacted with miR-494 to repress bladder cancer progression through targeting PTEN. J Cell Physiol 2020;235:1120-8. [Crossref] [PubMed]
- Feng SQ, Zhang XY, Fan HT, et al. Up-regulation of LncRNA MEG3 inhibits cell migration and invasion and enhances cisplatin chemosensitivity in bladder cancer cells. Neoplasma 2018;65:925-32. [Crossref] [PubMed]
- Lv D, Sun R, Yu Q, et al. The long non-coding RNA maternally expressed gene 3 activates p53 and is downregulated in esophageal squamous cell cancer. Tumour Biol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Kruer TL, Dougherty SM, Reynolds L, et al. Expression of the lncRNA Maternally Expressed Gene 3 (MEG3) Contributes to the Control of Lung Cancer Cell Proliferation by the Rb Pathway. PLoS One 2016;11:e0166363. [Crossref] [PubMed]
- Luo G, Wang M, Wu X, et al. Long Non-Coding RNA MEG3 Inhibits Cell Proliferation and Induces Apoptosis in Prostate Cancer. Cell Physiol Biochem 2015;37:2209-20. [Crossref] [PubMed]
- Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer 2018;18:5-18. [Crossref] [PubMed]
- Huang C, Liao X, Jin H, et al. MEG3, as a Competing Endogenous RNA, Binds with miR-27a to Promote PHLPP2 Protein Translation and Impairs Bladder Cancer Invasion. Mol Ther Nucleic Acids 2019;16:51-62. [Crossref] [PubMed]
- Paraskevopoulou MD, Hatzigeorgiou AG: Analyzing MiRNA-LncRNA Interactions. In: Feng Y, Zhang L. editors. Long Non-Coding RNAs: Methods and Protocols. New York: Springer, 2016:271-86
- Militello G, Weirick T, John D, et al. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief Bioinform 2017;18:780-8. [PubMed]
- Qin N, Tong GF, Sun LW, et al. Long Noncoding RNA MEG3 Suppresses Glioma Cell Proliferation, Migration, and Invasion by Acting as a Competing Endogenous RNA of miR-19a. Oncol Res 2017;25:1471-8. [Crossref] [PubMed]
- He JH, Han ZP, Liu JM, et al. Overexpression of Long Non-Coding RNA MEG3 Inhibits Proliferation of Hepatocellular Carcinoma Huh7 Cells via Negative Modulation of miRNA-664. J Cell Biochem 2017;118:3713-21. [Crossref] [PubMed]
- Du J, Liu S, He J, et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget 2015;6:14993-5007. [Crossref] [PubMed]
- Li C, Lyu J, Meng QH. MiR-93 Promotes Tumorigenesis and Metastasis of Non-Small Cell Lung Cancer Cells by Activating the PI3K/Akt Pathway via Inhibition of LKB1/PTEN/CDKN1A. J Cancer 2017;8:870-9. [Crossref] [PubMed]
- Jiang L, Wang C, Lei F, et al. miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway. Oncotarget 2015;6:8286. [PubMed]


