Clinical characteristics and treatments of large cell lung carcinoma: a retrospective study using SEER data
Introduction
Lung cancer is the most common malignant tumor in the world and has the highest fatality rate (1). It consists of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Histologically, NSCLC can be further classified as adenocarcinoma, squamous cell carcinoma and large cell carcinoma with or without neuroendocrine features. In NSCLC, according to cohort demographics and classification scheme, large cell lung cancer (LCLC) only accounts for 9% of all cases and often has poor differentiation and prognosis (2-5). Based on the WHO lung cancer classification (6), LCLC is defined as an undifferentiated non-small cell carcinoma, because it lacks the cellular and structural characteristics related to the adenocarcinoma or squamous cell carcinoma. The diagnosis of LCLC mainly depends on post-operative pathological examination rather than biopsy and cytological examination (7). There is evidence showing that LCLC is frequently found in males and smokers and often presents as a large mass with central necrosis (8-10). Furthermore, LCLC is more commonly seen in the elderly (>60), and its clinical symptoms and signs correlate with the location and the extent of invasion.
Due to its low incidence and a lack of relevant clinical data, less is known about its clinical and biological characteristics. In this retrospective study, the clinical information of patients with LCLC registered in the Surveillance, Epidemiology and End Results (SEER) database was extracted and analyzed, aiming to better understand its clinical behaviors and factors affecting the survival of patients.
Methods
Data extraction
The SEER database includes the information on cancer incidence and survival from 18 cancer registries, covering 26% of the population. Data for patients diagnosed with LCLC during 2004–2015 were extracted using the SEER*Stat software version 8.3.5. The study cohort contained patients according to the International Classification of Disease for Oncology, third edition (ICD-O-3) histology code 8012/3 (Large cell carcinoma, NOS), 8013/3 (Large cell neuroendocrine carcinoma), and 8014/3 (Large cell carcinoma with rhabdoid phenotype). Based on the 2004 WHO classification, large cell neuroendocrine carcinoma belongs to the family of large cell carcinoma. Therefore, such patients were also comprised in the cohort. The exclusion criteria were as follows: (I) patients had more than one primary tumors; (II) patients had no data on the survival; (III) the diagnosis was not pathologically confirmed; (IV) patients without clinicopathological information, including age, gender, race, marital status, primary site, laterality, grade, size, AJCC stage, chemotherapy and surgery. The clinical staging was determined according to the eighth TNM edition through the R version 3.4.3 software.
The outcomes included overall survival (OS) and lung cancer-specific survival (LCSS). The analysis cut-off date was 31 December 2015. OS was defined as the time from diagnosis to death from any cause or until the most recent follow-up. And the LCSS was defined as the time from diagnosis to either death caused by disease or last follow-up. In the SEER database, patients who survived for less than 1 month were coded with a survival time of zero. Therefore, a survival of 0.5 months was assigned to these patients following the standard epidemiological convention.
Statistical analysis
Categorical variables were analyzed with the Chi-square test. To adjust for the bias between surgical patients with or without chemotherapy, the propensity-matched (PSM) analysis was adopted. The PSM module was based upon age, gender, race, marital status, primary site, laterality, grade, size, and stage. Cumulative survival curves were determined using the Kaplan-Meier method and compared using the log-rank test. To perform univariate and multivariate analyses, Cox proportional hazards model was employed. Only variables that were significantly associated with the survival in univariate Cox analysis were included in the multivariate Cox analysis. Hazard ratio (HR) and 95% confidence interval (CI) were presented. And the nomogram was delineated based on the results of multivariate Cox analysis by using R version 3.4.3 software. The prediction error was estimated with 1000 bootstrap samples. A value of two-sided P<0.05 was considered statistically significant. Statistical analysis was performed with the software R version 3.4.3 and SPSS 25.0 (SPSS, Chicago, IL). GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) was used to delineate the survival curve.
Results
Clinical characteristics
As shown in Table 1, a total of 4,008 patients were diagnosed with LCLC between 2004 and 2015, and 70.2% of patients were older than 60 years. Slightly more than half of the patients were male (57.6%). In addition, the majority (53.9%) was married and 58.1% of tumors located in the right lung (41.4% in the left lung). Interestingly, the upper lobe was the most common site of lesions (60.2%), followed by the lower lobe (24.9%). And 97.8% of tumors showed poor differentiation or undifferentiation. Correspondingly, stage III/IV tumors accounted for 57.4%, while 20.1% and 12.5% of tumors were diagnosed at stage I and II, respectively. Moreover, the size of most tumors (62.4%) was less than 5 cm.
Table 1
| Characteristics | Number of cases (%) |
|---|---|
| Total | 4,008 |
| Age | |
| ≤60 | 1,196 (29.8) |
| >60 | 2,812 (70.2) |
| Gender | |
| Female | 1,699 (42.4) |
| Male | 2,309 (57.6) |
| Race | |
| White | 3,273 (81.7) |
| Black | 538 (13.4) |
| Other (American Indian/AK Native, Asian/Pacific Islander) | 197 (4.9) |
| Marital Status | |
| Married | 2,161 (53.9) |
| Single | 566 (14.1) |
| Other (Separated/Divorced/Widowed) | 1281 (32) |
| Primary site | |
| Main bronchus | 159 (4) |
| Upper lobe, lung | 2,411 (60.2) |
| Middle lobe, lung | 188 (4.7) |
| Lower lobe, lung | 998 (24.9) |
| Overlapping lesion | 56 (1.4) |
| Lung, NOS | 196 (4.9) |
| Laterality | |
| Left | 1,658 (41.4) |
| Right | 2,328 (58.1) |
| Bilateral | 22 (0.5) |
| Grade | |
| Well/Moderate | 89(2.2) |
| Poor/Undifferentiated | 3,919(97.8) |
| Tumor size (cm) | |
| ≤3 | 1,327 (33.1) |
| 3–5 | 1,174 (29.3) |
| 5–7 | 718 (17.9) |
| ≥7 | 789 (19.7) |
| Stage | |
| I | 807 (20.1) |
| II | 501 (12.5) |
| III | 966 (24.1) |
| IV | 1,734 (43.3) |
| Chemotherapy | |
| No/Unknown | 2,181 (54.4) |
| Yes | 1,827 (45.6) |
| Surgery | |
| No/Unknown | 2,364 (59) |
| Yes | 1,644 (41) |
Treatments
Table 2 shows the relevant treatments of LCLC. Only 26.1% of patients received surgery alone, while 15.0% of cases accepted surgery combined with chemotherapy. There were meaningful differences in the age, marital status, tumor size and stage between surgical patients with or without chemotherapy (P<0.05 for all). Neither distribution of gender, race, primary site, laterality nor grade differed significantly between objects who selected surgery or surgery plus chemotherapy. Furthermore, the results of the correlational analysis presented that with the improvement of size and stage, patients were more inclined to choose comprehensive treatment.
Table 2
| Characteristics | Chemotherapy | P value | |
|---|---|---|---|
| No/unknown (n=1,045) | Yes (n=599) | ||
| Age | <0.01 | ||
| ≤60 | 250 (23.9) | 279 (46.6) | |
| >60 | 795 (76.1) | 320 (53.4) | |
| Gender | 0.744 | ||
| Female | 478 (45.7) | 269 (44.9) | |
| Male | 567 (54.3) | 330 (55.1) | |
| Race | 0.474 | ||
| White | 886 (84.8) | 506 (84.5) | |
| Black | 101 (9.7) | 66 (11) | |
| Other (American Indian/AK Native, Asian/Pacific Islander) | 58 (5.6) | 27 (4.5) | |
| Marital status | 0.014 | ||
| Married | 582 (55.7) | 372 (62.1) | |
| Single | 124 (11.9) | 73 (12.2) | |
| Other (separated/divorced/widowed) | 339 (32.4) | 154 (25.7) | |
| Primary site | 0.348 | ||
| Main bronchus | 9 (0.9) | 5 (0.8) | |
| Upper lobe, lung | 667 (63.8) | 400 (66.8) | |
| Middle lobe, lung | 56 (5.4) | 21 (3.5) | |
| Lower lobe, lung | 281 (26.9) | 150 (25) | |
| Overlapping lesion | 15 (1.4) | 14 (2.3) | |
| Lung, NOS | 17 (1.6) | 9 (1.5) | |
| Laterality | 0.835 | ||
| Left | 462 (44.2) | 268 (44.7) | |
| Right | 583 (55.8) | 331 (55.3) | |
| Bilateral | 0 | 0 | |
| Grade | 0.442 | ||
| Well + moderate | 37 (3.5) | 17 (2.8) | |
| Poor + undifferentiated | 1,008 (96.5) | 582 (97.2) | |
| Tumor size (cm) | <0.01 | ||
| ≤3 | 573 (54.8) | 201 (33.6) | |
| 3–5 | 282 (27) | 216 (36.1) | |
| 5–7 | 112 (10.7) | 95 (15.9) | |
| ≥7 | 78 (7.5) | 87 (14.5) | |
| Stage | <0.01 | ||
| I | 594 (56.8) | 110 (18.4) | |
| II | 211 (20.2) | 184 (30.7) | |
| III | 153 (14.6) | 225 (37.6) | |
| IV | 87 (8.3) | 80 (13.4) | |
Survival
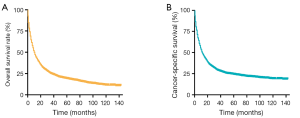
The median OS and LCSS were 35 months (95% CI: 30.61–39.40) and 55 months (95% CI: 43.29–66.71), respectively. Figure 1 show the OS and LCSS curves. Meanwhile, the overall 1-, 3- and 5-year survival rates were 45.6%, 24.8%, and 19.0%. Correspondingly, the 1-, 3-, and 5-year of LCSS were 49.1%, 28.8% and 24.1%, respectively. In addition, the median survival time was 34 months (range: 28.59–39.41 months) in patients undergoing surgery alone and 38 months (range: 31.04–44.99 months) in those receiving surgery plus chemotherapy (Figure 2A). Moreover, in patients with surgery alone and those with surgery combined with chemotherapy, the 1-year survival rate was 71.7% and 77.5%; the 3-year survival rate was 48.3 and 50.7%; and the 5-year survival rate was 38.8% and 41.0%, separately. As shown in Figure 2B, the OS in patients receiving surgery with chemotherapy was better than in patients undergoing surgery alone (P=0.041, HR =0.875, 95% CI: 0.771–0.993).
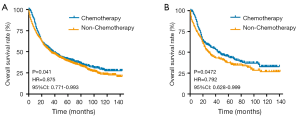
Furthermore, the PSM analysis was done in surgical patients with or without chemotherapy based on the age, gender, race, marital status, primary site, laterality, grade, tumor size, and stage. In the analysis, 244 patients received chemotherapy and 244 subjects had no chemotherapy (1:1). There were no significant differences in the clinical characteristics between them (Table S1). OS curves and Log-rank analysis indicated that patients receiving chemotherapy enhanced the survival (P=0.047, HR=0.7924, 95% CI: 0.628–0.999).
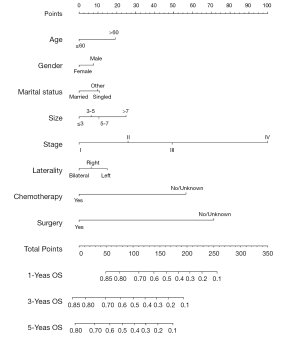
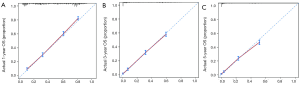
Variables potentially influencing OS were further investigated using the univariate Cox proportional hazards analysis. Table 3 displays the potential factors (P<0.01) associated with the prognosis except for the race (P=0.18). Further multivariate Cox analysis was performed to identify the independent prognostic elements. Results showed age (P<0.01), gender (P=0.01), marital status (P=0.004), laterality (P=0.015), tumor size (P<0.01), stage (P<0.01), chemotherapy (P<0.01) and surgery (P<0.01) were able to predict the survival of LCLC patients. The pathological grade correlated with the stage, thus it could exclude. Moreover, a nomogram (Figure 3) was-plotted based on the risk factors identified by the multivariate analysis for predicting 1-, 3-, and 5-year OS. And according to the internal bootstrap resampling validation, the calibration plot (Figure 4) was illustrated. The C-index for prediction of OS was 0.757 (95% CI: 0.749–0.765), which indicated a sufficient level of discrimination.
Table 3
| Independent variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | <0.01 | <0.01 | |||
| ≤60 | 1.00 (reference) | 1.00 (reference) | |||
| >60 | 1.252 (1.159–1.353) | 1.264 (1.166–1.371) | |||
| Gender | <0.01 | 0.01 | |||
| Female | 1.00 (reference) | 1.00 (reference) | |||
| Male | 1.184 (1.103–1.270) | 1.101 (1.023–1.186) | |||
| Race | 0.18 | ||||
| White | 1.00 (reference) | ||||
| Black | 1.04 (0.939–1.152) | 0.446 | |||
| Other (American Indian/AK Native, Asian/Pacific Islander) | 0.875 (0.744–1.029) | 0.106 | |||
| Marital Status | <0.01 | 0.004 | |||
| Married | 1.00 (reference) | 1.00 (reference) | |||
| Single | 1.192 (1.074–1.322) | 0.001 | 1.136 (1.021–1.264) | 0.019 | |
| Other (separated/divorced/widowed) | 1.126 (1.043–1.216) | 0.002 | 1.125 (1.038–1.219) | 0.004 | |
| Primary site | <0.01 | ||||
| Main bronchus | 1.00 (reference) | ||||
| Upper lobe, lung | 0.588 (0.497–0.695) | <0.01 | |||
| Middle lobe, lung | 0.613 (0.490–0.769) | <0.01 | |||
| Lower lobe, lung | 0.631 (0.529–0.752) | <0.01 | |||
| Overlapping lesion | 0.504 (0.354–0.718) | <0.01 | |||
| Lung, NOS | 1.147 (0.923–1.426) | 1.147 | |||
| Laterality | 0.004 | 0.015 | |||
| Left | 1.00 (reference) | 1.00 (reference) | |||
| Right | 0.974 (0.908–1.045) | 0.463 | 0.903 (0.841–0.969) | 0.005 | |
| Bilateral | 2.037 (1.310–3.169) | 0.002 | 0.822 (0.527–1.283) | 0.388 | |
| Grade | 0.012 | ||||
| Well + moderate | 1.00 (reference) | ||||
| Poor + undifferentiated | 1.37 (1.072–1.752) | ||||
| Tumor size (cm) | <0.01 | <0.01 | |||
| ≤3 | 1.00 (reference) | 1.00 (reference) | |||
| 3–5 | 1.332 (1.218–1.457) | <0.01 | 1.081 (0.983–1.188) | 0.107 | |
| 5–7 | 1.62 (1.463–1.794) | <0.01 | 1.134 (1.015–1.267) | 0.026 | |
| ≥7 | 2.186 (1.981–2.412) | <0.01 | 1.346 (1.208–1.500) | <0.01 | |
| Stage | <0.01 | <0.01 | |||
| I | 1.00 (reference) | 1.00 (reference) | |||
| II | 1.277 (1.110–1.469) | 0.001 | 1.386 (1.192–1.611) | <0.01 | |
| III | 2.052 (1.829–2.301) | <0.01 | 1.858 (1.619–2.132) | <0.01 | |
| IV | 4.831 (4.348–5.367) | <0.01 | 3.454 (3.018–3.953) | <0.01 | |
| Chemotherapy | <0.01 | <0.01 | |||
| No/unknown | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 0.852 (0.794–0.913) | 0.504 (0.467–0.543) | |||
| Surgery | <0.01 | <0.01 | |||
| No/unknown | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 0.282 (0.261–0.305) | 0.411 (0.373–0.453) | |||
Discussion
According to the 2004 lung cancer classification (11), LCLC is a poorly differentiated tumor and accounts for about 10% of NSCLC. However, with the emergence of 2015 lung cancer classification (6), basaloid carcinoma is classified as squamous cell carcinoma, the large cell neuroendocrine carcinoma and compound large cell neuroendocrine carcinoma are classified as neuroendocrine tumors, the lymphoepithelioma-like carcinoma is categorized as other or unclassified cancer, finally, the clear cell carcinoma and LCLC with rhabdoid phenotype are not comprised. Thus, the incidence of LCLC is likely to be lower than 10%, based on the new classification (9).
In our series, LCLC was more common in males and in the elderly at the time of diagnosis. In addition, lesions predominated on upper lobe, which was consistent with previous findings (12-14). Cao et al. also reported that large cell neuroendocrine carcinoma frequently observed in men and old people (15). Cao et al. (15) and Oshiro et al. (16) held that most of large cell neuroendocrine carcinoma was pathologically high-grade. In our study, 97.8% of patients showed poor differentiation at the time of diagnosis. Previous researches appeared that LCLC formed a large mass (13,17). During our research, nearly half of the neoplasms was smaller than 5 cm in diameter.
Early diagnosis and early treatment are crucial for the survival of LCLC patients because of the poor prognosis. A prospective study on large cell neuroendocrine carcinoma showed that postoperative chemotherapy could achieve a better prognosis as compared to surgery alone (18). In addition, Kujtan et al. found that the survival time of patients with stage IA large cell neuroendocrine carcinoma who received surgery combined with chemotherapy was significantly longer than in those receiving surgery alone (19). Hanagiri et al. presented that the 5-year survival rate in patients receiving surgery for large cell carcinoma was 61.5% (13). However, the 5-year survival rate was only 38.8% in our study. This might be ascribed to the small sample size (57 patients). Moreover, our results showed the 5-year survival rate of patients receiving surgery combined with chemotherapy was 41%, which was better than that of patients receiving surgery alone. Some studies had also recommended a combination of surgery and chemotherapy for the treatment of LCLC, and post-operative cisplatin/pemetrexed may achieve a better prognosis than post-operative cisplatin/gemcitabine (10.4 vs. 6.7 months, respectively) (20).
There were also several limitations in our study. First, we carried out the research according to the 2004 lung cancer classification, which is different from the 2015 lung cancer classification. Second, patients were divided into two groups according to whether they received chemotherapy or not, but the specific regimens for chemotherapy were unknown. At last, for patients diagnosed with LCLC, more prospective clinical studies are needed to elucidate the prognostic factors and further investigate the efficacy of available treatments.
In conclusion, LCLC is more common in the elderly (>60 years) and mainly located in the upper lobe. The majority of tumors are diagnosed at stage III/IV. Surgery combined with chemotherapy is beneficial for the prognosis of LCLC patients.
Table S1
| Characteristics | Non-chemotherapy (n=244) | Chemotherapy (n=244) | P value |
|---|---|---|---|
| Age | 1 | ||
| ≤60 | 69 | 69 | |
| >60 | 175 | 175 | |
| Gender | 1 | ||
| Female | 98 | 98 | |
| Male | 146 | 146 | |
| Race | 1 | ||
| White | 228 | 228 | |
| Black | 11 | 11 | |
| Other (American Indian/AK Native, Asian/Pacific Islander) | 5 | 5 | |
| Marital Status | 1 | ||
| Married | 175 | 175 | |
| Single | 14 | 14 | |
| Other (separated/divorced/widowed) | 55 | 55 | |
| Primary site | 1 | ||
| Main bronchus | 0 | 0 | |
| Upper lobe, lung | 174 | 174 | |
| Middle lobe, lung | 9 | 9 | |
| Lower lobe, lung | 59 | 59 | |
| Overlapping lesion | 1 | 1 | |
| Lung, NOS | 1 | 1 | |
| Laterality | 1 | ||
| Left | 112 | 112 | |
| Right | 132 | 132 | |
| Bilateral | 0 | 0 | |
| Grade | 1 | ||
| Well + moderate | 0 | 0 | |
| Poor + undifferentiated | 244 | 244 | |
| Tumor size (cm) | 1 | ||
| ≤3 | 83 | 83 | |
| 3–5 | 96 | 96 | |
| 5–7 | 37 | 37 | |
| ≥7 | 28 | 28 | |
| Stage | 1 | ||
| I | 80 | 80 | |
| II | 95 | 95 | |
| III | 55 | 55 | |
| IV | 14 | 14 |
Acknowledgments
Funding: Supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Kupeli M, Koseoglu RD. Large Cell Carcinoma with Adenocarcinoma in Lung. J Coll Physicians Surg Pak 2018;28:240-2. [Crossref] [PubMed]
- Hou LK, Zhang LP, Zhang W, et al. Clinicopathologic features and genetic profile of the redefined large cell lung carcinoma. Zhonghua Bing Li Xue Za Zhi 2017;46:298-302. [PubMed]
- Rekhtman N, Tafe LJ, Chaft JE, et al. Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod Pathol 2013;26:511-22. [Crossref] [PubMed]
- Chan AW, Chau SL, Tong JH, et al. The Landscape of Actionable Molecular Alterations in Immunomarker-Defined Large-Cell Carcinoma of the Lung. J Thorac Oncol 2019;14:1213-22. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Pelosi G, Barbareschi M, Cavazza A, et al. Large cell carcinoma of the lung: a tumor in search of an author. A clinically oriented critical reappraisal. Lung Cancer 2015;87:226-31. [Crossref] [PubMed]
- Copin MC. Large cell carcinoma, lymphoepithelioma-like carcinoma, NUT carcinoma. Ann Pathol 2016;36:24-33. [Crossref] [PubMed]
- Bi Y, Qu Y, Liang Z, et al. Clinicopathological analysis of Large Cell Lung Carcinomas definitely diagnosed according to the New World Health Organization Criteria. Pathol Res Pract 2018;214:555-9. [Crossref] [PubMed]
- Weissferdt A. Large cell carcinoma of lung: On the verge of extinction? Semin Diagn Pathol 2014;31:278-88. [Crossref] [PubMed]
- Lantuéjoul S, Brambilla E. What's new in the 2004 WHO classification of the lung tumors?. Rev Pneumol Clin 2008;64:187-94. [PubMed]
- Sun YH, Lin SW, Hsieh CC, et al. Treatment outcomes of patients with different subtypes of large cell carcinoma of the lung. Ann Thorac Surg 2014;98:1013-9. [Crossref] [PubMed]
- Hanagiri T, Oka S, Takenaka S, et al. Results of surgical resection for patients with large cell carcinoma of the lung. Int J Surg 2010;8:391-4. [Crossref] [PubMed]
- Gálffy G. Diagnosis and treatment of the neuroendocrine tumors of the lung. Magy Onkol 2018;62:113-8. [PubMed]
- Cao L, Li ZW, Wang M, et al. Clinicopathological characteristics, treatment and survival of pulmonary large cell neuroendocrine carcinoma: a SEER population-based study. Peer J 2019;7:e6539. [Crossref] [PubMed]
- Oshiro Y, Kusumoto M, Matsuno Y, et al. CT findings of surgically resected large cell neuroendocrine carcinoma of the lung in 38 patients. AJR Am J Roentgenol 2004;182:87-91. [Crossref] [PubMed]
- Park MS, Shin DH, Chung KY, et al. Clinical features of bronchogenic large cell carcinoma confirmed by surgical resection. Korean J Intern Med 2003;18:212-9. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802-7. [Crossref] [PubMed]
- Kujtan L, Muthukumar V, Kennedy KF, et al. The Role of Systemic Therapy in the Management of Stage I Large Cell Neuroendocrine Carcinoma of the Lung. J Thorac Oncol 2018;13:707-14. [Crossref] [PubMed]
- Tiseo M, Bartolotti M, Gelsomino F, et al. First-line treatment in advanced non-small-cell lung cancer: the emerging role of the histologic subtype. Expert Rev Anticancer Ther 2009;9:425-35. [Crossref] [PubMed]