Atezolizumab-induced psoriasis in a patient with metastatic lung cancer—a case report
Introduction
Immune checkpoint inhibitors have emerged as a prominent treatment choice for various cancers, such as non-small cell lung cancer (NSCLC), urothelial carcinoma (UCC), renal cell carcinoma (RCC), head and neck squamous cell carcinoma (HNSCC) and others (1). Although the immune checkpoint inhibitors have the impressive clinical benefits, these treatments can also cause varied of immune-related adverse events (2). Cutaneous toxicities are most common toxicities induced by immune checkpoint inhibitors (3,4).
However, cases of immune checkpoint inhibitors induced psoriasis have been reported rarely. As so far, there are only 4 cases presenting anti-programmed death ligand 1 (anti-PD-L1) induced psoriasis, in which two cases receive atezolizumab (5,6), and another two receive durvalumab (7). All of patients are from non-Asia areas. We report a case of a psoriasis flare with anti-PD-L1 treatment for lung cancer.
Case presentation
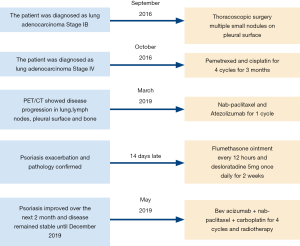
A 53-year-old Chinese man with a 20 pack-year smoking history presented with symptoms of cough and scant hemoptysis. A mass in upper right lung was found by chest computed tomography, and it was identified as adenocarcinoma by testing bronchoscopic biopsy specimen. No distance metastasis was observed in positron emission tomography/computed tomography (PET/CT). Stage IB (T2aN0M0) adenocarcinoma was diagnosed in September 2016. However, multiple small nodules on the pleural surface were found during thoracoscopic surgery and right upper lobectomy + mediastinal lymph node dissection of the pulmonary hilum + pleural nodule ablative therapy were performed. Stage IV (T2aN2M1a) adenocarcinoma was diagnosed postoperatively. Genetic testing suggested that sensitizing epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) rearrangement, high-level mesenchymal-epithelial transition (MET) amplification or MET exon 14 skipping mutation, ERBB2 mutations, ROS1 rearrangement, RET rearrangement, BRAF V600E mutation and KRAS mutations were negative. This patient had completed pemetrexed/cisplatin for 4 cycles and no clinical recurrence was observed.
Medical history shows this patient had psoriasis for 21 years without systemic therapy in recent 5 years and sulfa allergy presenting with red rash, pruritus and cyanosis. He also had undergone gallstone excision in 2008. There was no relevant family history or psycho-social history.
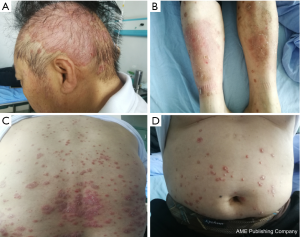
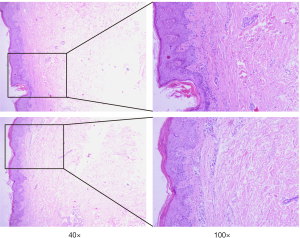
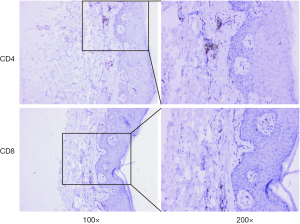
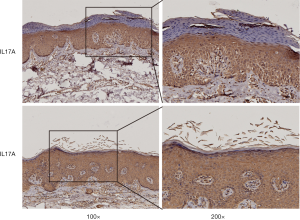
Unfortunately, there was unequivocal disease progression in March 2019, which PET/CT showed extensive hypermetabolic mass in the right upper lobe, extensive hypermetabolic right hilar lymph nodes, multiple small nodules on the right pleural surface and bone destruction in the 10th rib. Pleural biopsy revealed adenocarcinoma and programmed death ligand 1 (PD-L1) negative. About 14 days after he receiving 1 cycle of nab-paclitaxel 235 mg + atezolizumab 1,200 mg, he developed scaly plaque-like skin lesions on his head and limbs, guttate lesions on the trunk (Figure 1), which were intensely pruritic. A skin punch biopsy was performed and revealed these were hyperkeratosis (Figure 2) with CD4 and CD8 negative (Figure 3). In addition, IL-17A expression was positive in skin biopsy specimen by immunohistochemistry staining (Figure 4). Psoriasis was treated with flumethasone ointment every 12 hours and desloratadine 5mg once daily for 2 weeks. Atezolizumab was discontinued because of the psoriasis flare. Given the skin toxicity of atezolizumab, he received chemotherapy (bevacizumab 500 mg d1 + nab-paclitaxel 200 mg d1, 100 mg d8 + carboplatin 500 mg d1) in 4 cycles and radiotherapy. A PET/CT performed revealed complete response of the pulmonary metastasis, lymph nodes, nodular pleural disease and bone metastasis after 2 cycles of treatment with chemotherapy. There was an overall improvement of the psoriasis over the next 2 months and the patient remained stable disease until December, 2019. The timeline of diagnosis, interventions and outcomes was summarized as Figure 5.
Discussion
Atezolizumab is a humanized, engineered immunoglobulin G1 monoclonal antibody targeting programmed death ligand 1, which plays an important role in suppressing the immune system triggered by disease. Atezolizumab blocks not only PD-L1 and PD-1 binding, but also the PD-L1 and CD80 interaction, and therefore it is different from other anti-PD-1 antibodies (8). Ateolizumab was approved by FDA for the treatment of patients with metastatic NSCLC. It recommended a fix dose of 1,200 mg administered as an intravenous infusion every 3 weeks. Importantly, atezolizumab has no influence on PD-L2 and PD-1, and may minimize autoimmunity (9).
Dermatologic toxicities occur in more than one-third of the patients treated with monoclonal anti-PD-1, anti-PD-L1 and monoclonal antibodies targeting cytotoxic T lymphocyte-associated antigen-4 (anti-CTLA-4) agents (10). The overall incidence of dermatologic toxicities is higher with anti-PD-1 compared to anti-PD-L1 agents (11). The profile of the dermatologic toxicity is very similar: skin rash, pruritus, vitiligo, autoimmune skin disorders and other cutaneous toxicities.
Immune checkpoint inhibitors remove inhibitory signals of T-cell activation leading to not only antitumor immunity but also autoimmunity. Keratinocytes have been established as one of the key components for psoriasis (12). It has been shown that PD-L1 is expressed on keratinocytes for mediating peripheral T cell tolerance by nonlymphoid cells (13,14). Moreover, expressions of PD-L1 mRNA and protein levels are significantly decreased in psoriatic epidermis (15). With the use of anti-PD-L1 inhibitor, the PD-L1 decreased on keratinocytes, which may contribute to its chronic unregulated inflammatory characteristics. Therefore, this is one of the potential mechanisms that psoriasis may develop after administering anti-PD-L1 inhibitors. In addition, there is evidence that interleukin (IL)-17, IL-21 and IL-22 which are CD4+ T cell (Th17)-related cytokine play an important role in the pathogenesis of psoriasis (16,17). Furthermore, immune checkpoint inhibitors not only activate the augmentation of Th1 and Th17 cell, but also stimulate ILs produced from these cells and lead to the exacerbation of psoriasis (18). It has been documented that IL-17-mediated inflammation plays a major in the pathogenesis of psoriasis (19). In this case, atezolizumab therapy resulted in IL-17A expression up-regulated and led to psoriasis triggered, suggesting that anti-PD-L1 destroy the immune balance in non-cancer tissues. The association of the IL-17A expression up-regulated on the lesions following anti-PD-L1 atezolizumab is supported by the study that recombinant PD-L1-Fc alleviates psoriatic inflammation in imiquimod-treated mice by suppressing IL-17A (20).
Several cases documented psoriasis flare with anti-PD-1 and anti-PD-L1 (5-7,21). Most of them receive anti-PD-1, only two cases receive atezolizumab (5,6), and another two receive durvalumab (7). It is relevant to highlight that the median delay between the introduction of anti-PD-1 therapy and psoriasis flare is 31 days (5), however, the median days of anti-PD-L1 therapy is 13 days. Our case had the psoriasis flare at 14 days, which was close to the median onset. Besides days of delay, the number of infusions until psoriasis flare of anti-PD-L1, 1 for three cases, 2 for one case, is less than anti-PD-1 (5,22). Our case had the psoriasis onset following the first infusion of atezolizumab, suggesting that initial use of anti-PD-L1 is more likely to induce psoriasis. All the cases using anti-PD-L1 including ours had a history of psoriasis, indicating that personal psoriasis-related history seems to be one of the significant risk factors. Phototherapies seem to be helpful for psoriasis and used in many cases, while our case used local corticosteroid and improved. However, there is no survival outcome of patients reported to perform further investigations between its development and the benefit of therapy (22).
There are several novel aspects to this case report. Firstly, it is the first case of anti-PD-L1 inhibitors induced-psoriasis from Asia area, while others are from Greece (7), France (5) and Spain (6), suggesting that there is no difference among races. Another distinctive aspect is that the psoriasis induced by Atezolizumab of this case was confirmed by a skin punch biopsy and IL-17A expression was positive in skin biopsy specimen by immunohistochemistry staining, showing that secukinumab, an IL-17 inhibitor, may be an option of therapy. Nevertheless, this case has several limitations, most notably the inaccessibility of an IL-17 inhibitor, which makes it difficult to continue anti-PD-L1 therapy. The co-administration of secukinumab and pembrolizumab seems no complications and no recurrence of NSCLC (23). Furthermore, the follow-up time was too short to assess the therapeutic effect.
Conclusions
Immune checkpoint inhibitors, especially anti-PD-1 and anti-PD-L1, do induce psoriasis exacerbation. Our case reports a 53-year-old Chinese man with NSCLC who presented with psoriasis after anti-PD-L1 atezolizumab initiation. A biopsy was performed to confirm the diagnosis of psoriasis, suggesting that atezolizumab suppressed PD-L1 expression on keratinocytes and IL-17A expression was up-regulated. It is important for oncologists to be aware of potential psoriasis exacerbation when patients use anti-PD-L1, especially who had a personal psoriasis-related history.
Acknowledgments
We thank our patient providing the information for us.
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.57). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent for the publication of the report, and the concomitant images, was provided by the patient. The submission version of the report was confirmed as being correct to the best of his knowledge. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang GX, Kurra V, Gainor JF, et al. Immune Checkpoint Inhibitor Cancer Therapy: Spectrum of Imaging Findings. Radiographics 2017;37:2132-44. [Crossref] [PubMed]
- Marin-Acevedo JA, Chirila RM, Dronca RS. Immune Checkpoint Inhibitor Toxicities. Mayo Clin Proc 2019;94:1321-9. [Crossref] [PubMed]
- Plachouri KM, Vryzaki E, Georgiou S. Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Summarized Overview. Curr Drug Saf 2019;14:14-20. [Crossref] [PubMed]
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol 2016;28:254-63. [Crossref] [PubMed]
- Bonigen J, Raynaud-Donzel C, Hureaux J, et al. Anti-PD1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol 2017;31:e254-7. [Crossref] [PubMed]
- Santos-Juanes J, Munguia Calzada P, Alvarez Fernandez C. Plaque Psoriasis Flare and Peripheral Edema in a Patient Treated With Atezolizumab. Actas Dermosifiliogr 2019;110:410-1. [Crossref] [PubMed]
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-PD1/PDL1 induced psoriasis. Curr Probl Cancer 2017;41:407-12. [Crossref] [PubMed]
- Markham A. Atezolizumab: First Global Approval. Drugs 2016;76:1227-32. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am J Clin Dermatol 2018;19:345-61. [Crossref] [PubMed]
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol 2017;44:158-76. [Crossref] [PubMed]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361:496-509. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95. [Crossref] [PubMed]
- Kim DS, Je JH, Kim SH, et al. Programmed death-ligand 1, 2 expressions are decreased in the psoriatic epidermis. Arch Dermatol Res 2015;307:531-8. [Crossref] [PubMed]
- Shi Y, Chen Z, Zhao Z, et al. IL-21 Induces an Imbalance of Th17/Treg Cells in Moderate-to-Severe Plaque Psoriasis Patients. Front Immunol 2019;10:1865. [Crossref] [PubMed]
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol 2014;71:141-50. [Crossref] [PubMed]
- Dulos J, Carven GJ, van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 2012;35:169-78. [Crossref] [PubMed]
- Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014;32:227-55. [Crossref] [PubMed]
- Kim JH, Choi YJ, Lee BH, et al. Programmed cell death ligand 1 alleviates psoriatic inflammation by suppressing IL-17A production from programmed cell death 1-high T cells. J Allergy Clin Immunol 2016;137:1466-76.e3. [Crossref] [PubMed]
- Chia PL, John T. Severe Psoriasis Flare After Anti-Programmed Death Ligand 1 (PD-L1) Therapy for Metastatic Non-Small Cell Lung Cancer (NSCLC). J Immunother 2016;39:202-4. [Crossref] [PubMed]
- Ruiz-Bañobre J, Garcia-Gonzalez J. Anti-PD-1/PD-L1-induced psoriasis from an oncological perspective. J Eur Acad Dermatol Venereol 2017;31:e407-8. [Crossref] [PubMed]
- Monsour EP, Pothen J, Balaraman R. A Novel Approach to the Treatment of Pembrolizumab-induced Psoriasis Exacerbation: A Case Report. Cureus 2019;11:e5824. [PubMed]