
Analysis of factors related to N2- or N3-stage breast cancer associated with 1–2 positive sentinel lymph nodes in Chinese patients
Introduction
Axillary lymph node metastasis status is an important prognostic factor for patients with breast cancer (1). Sentinel lymph node (SLN) biopsy has been widely used as a standard procedure for providing accurate tumor staging in patients with early-stage breast cancer (2-4), Axillary lymph node dissection (ALND) can be safely avoided in node-negative breast cancer patients (5-7). In 2007, the NSABP B-32 trial recommended routine ALND for patients with positive SLNs (6). In 2010, the ACOSOG Z0011 trial revealed that compared to SLN biopsy, ALND did not improve the overall or disease-free survival rate, or the locoregional recurrence rate, but did result in more complications in women with clinically node-negative, T1–T2 invasive breast cancer and one or two positive SLNs, who underwent breast-conservation surgery (8,9). Furthermore, the 2014 EORTC AMAROS trial reported that axillary-specific radiotherapy was equivalent to ALND among women with clinically node-negative, T1–T2 invasive breast cancer and positive SLNs, who were treated with either breast-conservation surgery or mastectomy (10). In addition, a recent update of the Z0011 trial data revealed that the 10-year local recurrence-free survival and overall survival rates for eligible patients treated with SLN dissection alone were non-inferior to the rates achieved after ALND (11,12). In a subsequent research published in 2018, Park et al. reported that ALND improved the survival of patients with cN2–N3 invasive breast cancer (13). On the basis of the above evidence, it was hypothesized that for women with clinically early-stage breast cancer and one or two positive SLNs, who underwent breast-conserving surgery, axillary dissection should be withheld for those with N1-stage disease. In contrast, those with N2- or N3-stage disease require ALND to improve their survival and prognosis. However, at present, it remains unclear whether SLN biopsy is sufficient for the entire cohort of patients with clinical early-stage breast cancer and one or two positive SLNs, including patients who were subsequently found to have N2- or N3-stage disease on the postoperative pathological examination. Previous studies (14-16) have focused on the prediction of non-SLN metastasis in a setting similar to that in the Z0011 trial, but few studies have investigated the alteration in the N stage after surgery.
Therefore, the present study determined the percentage of patients with pN2- or pN3-stage disease in a cohort of patients with T1–T2 invasive breast cancer and one or two positive SLNs. Furthermore, the risk factors for pN2- or pN3-stage disease in the present patient population were also identified.
Methods
Patients
The medical data of women with breast cancer, who underwent SLN biopsy in the First Affiliated Hospital of Guangxi Medical University between June 2011 and July 2018, were retrospectively reviewed. The medical, surgical and pathological data of these patients were obtained from electronic medical records. Breast cancer patients who had clinically negative axillary lymph nodes and underwent SLN biopsy were selected for the present study. Patients were excluded when they had negative SLNs, did not undergo ALND for any reason (e.g., comorbidity, age, or refusal to undergo the procedure), had three or more positive SLNs, had bilateral breast cancer or a stage T3 or higher primary tumor at the time of diagnosis, or had a history of neoadjuvant systemic therapy.
The study protocol was approved by the Medical Ethic Committee of First Affiliated Hospital of Guangxi Medical University (2019(KY-E-032)), and the need for individual consent was waived due to the retrospective nature of the present study.
Intraoperative SLN biopsy
Intraoperative lymphatic mapping was performed using the periareolar injection of a blue dye (methylthioninium chloride; Jumpcan Pharmaceutical Group Co., Taizhou, China). Before mastectomy/lumpectomy, an axillary incision made at 1 cm below the hair-bearing area, or an incision for the mastectomy/lumpectomy was used to harvest the SLNs indicated by the blue dye or methylene blue-stained lymphatic duct. Enlarged lymph nodes detected around the SLNs were also classified as SLNs.
Any SLN collected during the above surgical procedure was subjected to real-time frozen-section evaluation, followed by permanent paraffin slice assessment. For the intraoperative evaluation, one to six 5-µm-thick frozen sections were obtained by slicing at 2-mm intervals perpendicular to the long axis. Then, these were stained with hematoxylin and eosin, and analyzed. Intraoperative ALND was carried out when any SLN was found to be positive on the intraoperative frozen-section analysis. Postoperative permanent hematoxylin and eosin staining was routinely applied to confirm the results of the frozen-section evaluation. Three to six 4-µm-thick sections at intervals of 40 µm were dissected for the postoperative analysis. An additional CK-19 and EMA immunohistochemistry assessment was employed in cases where the results of the permanent staining were equivocal.
TNM staging
The T stage in these present patients was determined according to the TNM staging system for breast cancer proposed by the American Joint Committee on Cancer: T1 stage, maximum tumor diameter ≤20 mm; T2 stage, maximum tumor diameter between 20 and 50 mm. The pathological staging of lymph nodes depended on the number of metastatic lymph nodes identified in the pathological report: pN1, 1–3 metastatic axillary lymph nodes; pN2, 4–9 metastatic lymph nodes; pN3, 10 or more metastatic lymph nodes.
Statistical analysis
Data on multiple patient and tumor characteristics were collected, including age, tumor size, T stage, pathological tumor type, histological tumor grade, lymphovascular invasion (LVI), multifocality, type of surgery, estrogen receptor (ER) status, progesterone receptor (PR) status, number of positive SLNs, human epidermal growth factor receptor-2 (HER-2) status, Ki-67 status, molecular tumor type, number of negative SLNs, and metastatic SLN ratio (metastatic SLNs/total SLNs).
The distribution of continuous variables was analyzed using the Mann-Whitney U-test. The chi-square test or Fisher exact test was used for categorical variables. In the univariate analysis, variables with a P value <0.05 were included in the backward stepwise logistic regression analysis. Variables in the logistic regression that had a P value of <0.05 were identified as independent predictive factors. IBM SPSS statistics version 16.0 software (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analysis.
Results
Patient characteristics and the proportion of N2- or N3-stage disease
Among the 1,769 patients who underwent SLN biopsy at our hospital during the study period, 298 patients with T1–T2 tumors, clinically negative axillae, and one or two metastatic SLNs were included in the present study (Figure 1). The characteristics of these 298 patients are listed in Table 1. All patients were diagnosed with unilateral primary breast cancer and underwent surgical management (mastectomy, 217 patients; breast-conserving surgery, 81 patients). An additional axillary metastasis was postoperatively found in 113 patients (37.9%).
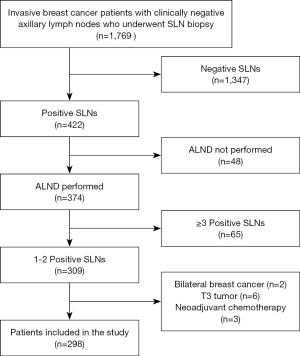
Table 1
| Variables | Total | N1 | N2/N3 | P value | χ2 (z) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||
| Patients | 298 | N/A | 250 | 83.9 | 48 | 16.1 | N/A | N/A | ||
| Median age [range] | 51 [21–82] | N/A | 47 [21–82] | N/A | 48 [33–65] | N/A | 0.978 | −0.27 (z) | ||
| Maximal tumor diameter (cm) | 2.337±1.010 | N/A | 2.263±0.932 | N/A | 2.725±1.290 | N/A | 0.021 | 12.921 (F) | ||
| T stage | ||||||||||
| T1 | 156 | 52.3 | 139 | 89.1 | 17 | 10.9 | 0.010 | 6.576 (χ2) | ||
| T2 | 142 | 47.7 | 111 | 78.2 | 31 | 21.8 | ||||
| Pathological tumor type | 0.502 | 1.376 (χ2) | ||||||||
| Invasive ductal carcinoma | 291 | 97.7 | 243 | 83.5 | 48 | 16.5 | ||||
| Invasive lobular carcinoma | 4 | 1.3 | 4 | 100 | 0 | 0 | ||||
| Other | 3 | 1.0 | 3 | 100 | 0 | 0 | ||||
| Histological tumor grade | 0.192 | 4.731 (χ2) | ||||||||
| G1 | 24 | 8.1 | 22 | 91.7 | 2 | 8.3 | ||||
| G2 | 185 | 62.1 | 152 | 82.2 | 33 | 17.8 | ||||
| G3 | 55 | 18.4 | 44 | 80.0 | 11 | 20.0 | ||||
| N/A | 34 | 11.4 | 32 | 94.1 | 2 | 5.9 | ||||
| Lymphovascular invasion | 0.000 | 28.053 (χ2) | ||||||||
| Present | 93 | 31.2 | 65 | 69.9 | 28 | 30.1 | ||||
| Absent | 205 | 68.8 | 185 | 90.2 | 20 | 9.8 | ||||
| Multifocality | 0.084 | 2.992 (χ2) | ||||||||
| Multifocal | 27 | 9.1 | 19 | 70.4 | 8 | 29.6 | ||||
| Unifocal | 271 | 90.9 | 231 | 85.2 | 40 | 14.8 | ||||
| Surgery | 0.280 | 1.165 (χ2) | ||||||||
| Conservative | 81 | 27.2 | 71 | 87.7 | 10 | 12.3 | ||||
| Mastectomy | 217 | 72.8 | 179 | 82.5 | 38 | 17.5 | ||||
| ER status | 0.646 | 0.210 (χ2) | ||||||||
| Positive | 237 | 79.5 | 200 | 84.4 | 37 | 15.6 | ||||
| Negative | 61 | 20.5 | 50 | 82.0 | 11 | 18.0 | ||||
| PR status | 0.646 | 0.210 (χ2) | ||||||||
| Positive | 225 | 75.5 | 190 | 84.4 | 35 | 15.6 | ||||
| Negative | 73 | 24.5 | 60 | 82.2 | 13 | 17.8 | ||||
| HER-2/neu receptor status | 0.973 | 0.055 (χ2) | ||||||||
| Positive | 120 | 40.3 | 100 | 83.3 | 20 | 16.7 | ||||
| Negative | 166 | 55.7 | 140 | 84.3 | 26 | 15.7 | ||||
| N/A | 12 | 4.0 | 10 | 83.3 | 2 | 16.7 | ||||
| Ki-67 status | 0.112 | 2.522 (χ2) | ||||||||
| ≤20% | 152 | 51.0 | 130 | 85.5 | 22 | 14.5 | ||||
| >20% | 146 | 49.0 | 120 | 82.2 | 26 | 17.8 | ||||
| Subtype | 0.876 | 1.804 (χ2) | ||||||||
| Luminal A | 56 | 18.8 | 50 | 89.3 | 6 | 10.7 | ||||
| Luminal B1 | 91 | 30.5 | 75 | 82.4 | 16 | 17.6 | ||||
| Luminal B2 | 87 | 29.2 | 73 | 83.9 | 14 | 16.1 | ||||
| HER2 | 33 | 11.1 | 27 | 81.8 | 6 | 18.2 | ||||
| TNBC | 19 | 6.4 | 15 | 78.9 | 4 | 21.1 | ||||
| N/A | 12 | 4.0 | 10 | 83.3 | 2 | 16.7 | ||||
| Mean total SLNs | 3.899±1.872 | N/A | 3.940±1.871 | N/A | 3.687±1.881 | N/A | 0.393 | 0.855 (F) | ||
| No. of positive SLNs | 0.000 | 16.173 (χ2) | ||||||||
| 1 | 204 | 68.5 | 183 | 89.7 | 21 | 10.3 | ||||
| 2 | 94 | 31.5 | 67 | 71.3 | 27 | 28.7 | ||||
| No. of negative SLNs | 0.075 | 12.890 (χ2) | ||||||||
| 0 | 32 | 10.7 | 21 | 65.6 | 11 | 34.4 | ||||
| 1 | 66 | 22.2 | 54 | 81.8 | 12 | 18.2 | ||||
| 2 | 57 | 19.1 | 48 | 84.2 | 9 | 15.8 | ||||
| 3 | 64 | 21.5 | 60 | 93.7 | 4 | 6.3 | ||||
| 4 | 39 | 13.1 | 33 | 84.6 | 6 | 15.4 | ||||
| 5 | 14 | 4.7 | 12 | 85.7 | 2 | 14.3 | ||||
| 6 | 15 | 5.0 | 13 | 86.7 | 2 | 13.3 | ||||
| ≥7 | 11 | 3.7 | 9 | 81.8 | 2 | 18.2 | ||||
| Metastatic SLN ratio | 0.000 | 18.593 (χ2) | ||||||||
| ≤0.5 | 245 | 82.2 | 216 | 88.2 | 29 | 11.8 | ||||
| >0.5, ≤1 | 53 | 17.8 | 34 | 64.2 | 19 | 35.8 | ||||
N/A, not applicable; ER, estrogen receptor; PR, progesterone receptor; TNBC, triple-negative breast cancer; SLN, sentinel lymph node.
The final N stage, as determined by postoperative pathological examination, was pN1 for 250 (83.9%) patients, and pN2 or pN3 for 48 (16.1%) patients (pN2, 11.41%; pN3, 4.70%). The median age of these patients was 47 years old (range, 21–82 years old) in the N1 group and 48 years old (range, 33–65 years old) in the N2/3 group. Among the 156 patients with T1 tumors, 17 (10.9%) patients had confirmed N2 or N3 disease, while among the 142 patients with T2 tumors, 31 (21.8%) patients had N2 or N3 disease.
Risk factors for N2- or N3-stage disease
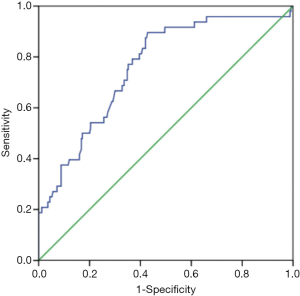
Based on the results of the univariate analysis, the following parameters had no association with the N stage: age, pathological tumor type, histological tumor grade, multifocality, type of surgery, ER status, PR status, HER-2 status, Ki-67 status, molecular tumor type, and the number of negative SLNs (P>0.05, Table 1). The variables that were significantly associated with a diagnosis of N2 or N3 stage were as follows: maximal tumor diameter, T2 stage, LVI, the number of positive SLNs, and the metastatic SLN ratio (P<0.05, Table 1). Since the maximal tumor diameter and T stage both represent tumor size, the T stage, which had a smaller P value in the univariate analysis, was first selected for inclusion in the multivariate analysis. This analysis identified the following as independent predictors of N2/3 disease: T2 stage, LVI, and the number of positive SLNs (P<0.05, Table 2). The area under the curve (AUC) for this model in the present patient series was 0.760 [95% confidence interval (CI), 0.689–0.831], suggesting acceptable discrimination (Figure 2). When the maximal tumor diameter was used to replace the T stage in the multivariate analysis, maximal tumor diameter, LVI and the number of positive SLNs were found to be independent predictors of N2/3 disease (P<0.05, Table 3). This is consistent with the results of a previous logistic regression model, which included the T stage. The AUC of the second model was 0.760 (95% CI: 0.688–0.833; Figure 3).
Table 2
| Variables | B | S.E. | Wald | df | Sig. | Exp (B) |
|---|---|---|---|---|---|---|
| Step 1a | ||||||
| LVI | 1.321 | 0.343 | 14.802 | 1 | 0.000 | 3.748 |
| T stage | 0.729 | 0.353 | 4.265 | 1 | 0.039 | 2.074 |
| Positive SLNs | 1.079 | 0.358 | 9.110 | 1 | 0.003 | 2.943 |
| Metastatic SLN ratio | 1.239 | 0.666 | 3.463 | 1 | 0.063 | 3.452 |
| Constant | −4.698 | 0.658 | 51.004 | 1 | 0.000 | 0.009 |
a, variable(s) entered in step 1: LVI, T stage, positive SLNs, metastatic SLN ratio. LVI, lymphovascular invasion; SLN, sentinel lymph node; S.E., standard error; df, degree of freedom; Sig., significance.
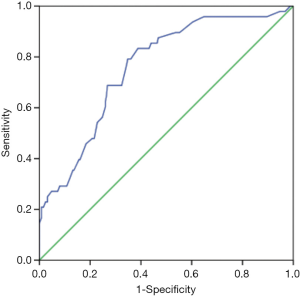
Table 3
| Variables | B | S.E. | Wald | df | Sig. | Exp (B) |
|---|---|---|---|---|---|---|
| Step 1a | ||||||
| LVI | 1.287 | 0.345 | 13.937 | 1 | 0.000 | 3.622 |
| Maximal tumor diameter | 0.318 | 0.157 | 4.089 | 1 | 0.043 | 1.375 |
| Positive SLNs | 1.064 | 0.357 | 8.850 | 1 | 0.003 | 2.897 |
| Metastatic SLN ratio | 1.241 | 0.668 | 3.449 | 1 | 0.063 | 3.459 |
| Constant | −5.053 | 0.741 | 46.467 | 1 | 0.000 | 0.006 |
a, variable(s) entered in step 1: LVI, maximal tumor diameter, positive SLNs, metastatic SLN ratio. LVI, lymphovascular invasion; SLN, sentinel lymph node; S.E., standard error; df, degree of freedom; Sig., significance.
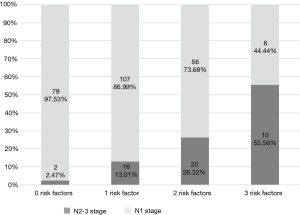
Subgroup analysis of the proportion of N2/3 disease based on risk factors
The proportions of N1 and N2/3 disease for patients with 0, 1, 2, or 3 risk factors were calculated (Figure 4). The rates of N2/3 disease in each of these patient subgroups were as follows: no risk factor, 2.47%; one risk factor, 13.01%; two risk factors, 26.32%; three risk factors, 55.56%.
Discussion
The results of the Z0011 and AMARAS trials revealed that ALND can be safely avoided in early-stage breast cancer patients with clinically negative axillary lymph nodes and two or fewer positive SLNs (8-10). Furthermore, the updated data obtained from the Z0011 trial confirmed that SLN biopsy alone is safe in terms of the long-term local recurrence-free survival and overall survival rates for selected early-stage breast cancer patients with one or two positive SLNs (11,12). It appears that the focus of the controversy on this topic has shifted from “the necessity of complete ALND in every patient with metastatic SLNs” to “the safety of avoiding ALND in every patient with positive SLNs.”
For patients with two or fewer metastatic SLNs, the risk of remnant non-SLN metastasis still exists and must be taken seriously, especially for patients in whom the N stage is up-staged after surgery. After all, no study has directly demonstrated that patients with N2 or N3 disease benefited from the exemption of ALND. On the contrary, a large retrospective study conducted by Park et al. in 2018 revealed that axillary lymphadenectomy was associated with improved survival in patients presenting with cN2/3 invasive breast cancer (13). From the above findings, the following inferences can be drawn: (I) patients with low tumor burden may benefit from the exemption of ALND, while (II) patients with a heavy axillary tumor burden need additional ALND to improve survival and prognosis. Thus, ALND may be safely withheld in patients with clinically axillary-negative early-stage breast cancer with one or two positive SLNs when the proportion of patients with a final pathological diagnosis of N2 or N3 disease is acceptable.
In 2017, a retrospective single-center trial conducted by Kim et al. (17) revealed that among 1,426 patients with T1/2 disease and one or two positive SLNs, 12.5% of patients had N2 or N3 metastasis. Furthermore, Liang et al. reported in 2019 that among the T1–2 cN0 breast cancer patients in their study, the nodal metastasis burden was significantly higher among those who underwent fine needle aspiration (n=202), when compared to patients who underwent SLN biopsy (n=186). However, even among those who underwent SLN biopsy, 6.49% (n=5) of patients with T1 tumors and 15.60% (n=17) of patients with T2 tumors had three or more axillary lymph node metastases (18). In the present study, 250 (83.89%) patients had pN1 stage lesions, and 48 (16.11%) patients had pN2 or pN3 stage lesions (11.41% had pN2 disease, and 4.70% had pN3 disease). A stratification analysis by T stage revealed that among the 156 patients with T1 tumors, 17 (10.90%) patients had pN2 or pN3 disease, while among the 142 patients in the T2 group, 31 (21.83%) patients had pN2 or pN3 disease.
The proportion of patients with N2 or N3 disease was higher than 10% in both the present study population and research cohort of Kim et al. (17), and this proportion was more than 20% in the T2 subgroup of the present study. Therefore, it is very important to distinguish patients with N2/3 disease from those with N1 disease. Hence, univariate and multivariate analyses were performed to evaluate the risk factors for N2/3 disease in the present study cohort. It was found that T2 stage, LVI and the number of positive SLNs were independent predictors of N2/3 disease.
The association of tumor size with the probability of non-SLN metastasis has been documented in numerous studies (19-22). In the present study, N2/3 disease was correlated to T2 stage. Furthermore, N2/3 disease was twice as common in the T2 group (21.83%), when compared to the T1 group (10.90%), which is consistent with the results reported by Kim et al. (17) However, it should be noted that the proportion of T1 tumors considerably varies among different clinical studies, which range from 10% to 70% (23-27). Furthermore, the differences in the ratio of T1 tumors may produce different proportions of N2 or N3 disease, which might affect the prognosis of this population. In the Z0011 trial (8,9), approximately 70% of patients who underwent SLN biopsy alone had T1 disease, and the median tumor size in this cohort was 1.6 cm. In the AMAROS trial (10), the median tumor size was 1.7 cm in the radiotherapy-alone group, which is similar to that in the previous cohort in the Z0011 trial. However, in the present study, approximately 50% of patients had T1 disease (the remaining patients had T2 tumors), and the median tumor size was 2.5 cm. Thus, patients in the present study had a more advanced T stage and larger tumor size. If previous trials had included more patients with higher tumor burden, the survival results would have been affected (17).
Some investigations have revealed that LVI is a predictor of non-SLN metastasis (21,27-29). Sandoughdaran et al. suggested that LVI is a predictor of axillary lymph node metastasis in women with early-stage breast cancer (30). In the present study, the authors reported the association between LVI and N2/3 lymph node involvement, which is consistent with the research reported by Kim et al. (17).
Some investigations have demonstrated that the number of positive SLNs is an important factor that predicts additional nodal involvement (14). Castrucci et al. considered that nodal ratio is more accurate in predicting the risk of regional recurrence (31). Both the nodal ratio and number of positive SLNs were associated with the N2/3 disease in the univariate analysis performed in the present study, which is consistent with the research reported by Kim et al. (17). In the study conducted by Kim et al., nodal ratio, rather than the number of positive SLNs, was included in the logistic regression analysis, and this was found to be an independent predictor of N2/3 lymph node involvement. However, in the present study, the number of positive SLNs was an independent predictor of N2/3 disease, rather than the nodal ratio, in the multivariate analysis.
Several limitations in the present study should be noted. The main limitation of the present study is the retrospective nature of the analysis, in which the data were subject to bias. Second, the present study has a single-center design, and the small number of included patients may not represent the whole population.
Conclusions
The present study revealed that N2/3 lymph node metastasis occurs in patients with T1–2 breast cancer and one or two positive SLNs, particularly in the T2 group. The proportion of patients with N2/3 disease was not negligible. T2 stage, LVI and the number of positive SLNs were the independent predictors of N2/3 lymph node metastasis in the present patient population.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.28). ML reports grants from Guangxi Zhuang Autonomous Region Science and Technology Department, during the conduct of the study; HY reports grants from Guangxi Zhuang Autonomous Region Science and Technology Department, during the conduct of the study; CL reports grants from Guangxi Zhuang Autonomous Region Science and Technology Department, during the conduct of the study; KS reports grants from Guangxi Zhuang Autonomous Region Science and Technology Department, during the conduct of the study; FL reports grants from Guangxi Zhuang Autonomous Region Science and Technology Department, during the conduct of the study; JZ reports grants from Guangxi Zhuang Autonomous Region Science and Technology Department, during the conduct of the study; of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Medical Ethic Committee of First Affiliated Hospital of Guangxi Medical University (2019(KY-E-032)) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The need for individual consent was waived due to the retrospective nature of the present study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983;52:1551-7. [Crossref] [PubMed]
- Turner RR, Ollila DW, Krasne DL, et al. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 1997;226:271-6; discussion 276-8. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997;349:1864-7. [Crossref] [PubMed]
- Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 2000;18:2553-9. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol 2006;7:983-90. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007;8:881-8. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama 2011;305:569-75. [Crossref] [PubMed]
- Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426-32; discussion 432-3. [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Giuliano AE, Ballman K, McCall L, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264:413-20. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Park TS, Thomas SM, Rosenberger LH, et al. The Association of Extent of Axillary Surgery and Survival in Women with N2-3 Invasive Breast Cancer. Ann Surg Oncol 2018;25:3019-29. [Crossref] [PubMed]
- Chen JY, Chen JJ, Xue JY, et al. Predicting Non-sentinel Lymph Node Metastasis in a Chinese Breast Cancer Population with 1-2 Positive Sentinel Nodes: Development and Assessment of a New Predictive Nomogram. World J Surg 2015;39:2919-27. [Crossref] [PubMed]
- Toshikawa C, Koyama Y, Nagahashi M, et al. Predictive Factors for Non-Sentinel Lymph Node Metastasis in the Case of Positive Sentinel Lymph Node Metastasis in Two or Fewer Nodes in Breast Cancer. J Clin Med Res 2015;7:620-6. [Crossref] [PubMed]
- Öz B, Akcan A, Dogan S, et al. Prediction of nonsentinel lymph node metastasis in breast cancer patients with one or two positive sentinel lymph nodes. Asian J Surg 2018;41:12-9. [Crossref] [PubMed]
- Kim I, Ryu JM, Kim JM, et al. Development of a Nomogram to Predict N2 or N3 Stage in T1-2 Invasive Breast Cancer Patients with No Palpable Lymphadenopathy. J Breast Cancer 2017;20:270-8. [Crossref] [PubMed]
- Liang Y, Chen X, Tong Y, et al. Higher axillary lymph node metastasis burden in breast cancer patients with positive preoperative node biopsy: may not be appropriate to receive sentinel lymph node biopsy in the post-ACOSOG Z0011 trial era. World J Surg Oncol 2019;17:37. [Crossref] [PubMed]
- Loza C M., Mando P, et al. Predictive Factors for Non-Sentinel Lymph Node Metastasis in Patients with ACOSOG Z0011 Criteria. Breast Care (Basel) 2018;13:434-8. [Crossref] [PubMed]
- Tapia G, Ying V, et al. Predicting non-sentinel lymph node metastasis in Australian breast cancer patients: are the nomograms still useful in the post-Z0011 era? ANZ J Surg 2019;89:712-7. [Crossref] [PubMed]
- Koca B, Kuru B, Ozen N, et al. A breast cancer nomogram for prediction of non-sentinel node metastasis - validation of fourteen existing models. Asian Pac J Cancer Prev 2014;15:1481-8. [Crossref] [PubMed]
- Canavese G, Bruzzi P, Catturich A, et al. A risk score model predictive of the presence of additional disease in the axilla in early-breast cancer patients with one or two metastatic sentinel lymph nodes. Eur J Surg Oncol 2014;40:835-42. [Crossref] [PubMed]
- Chen K, Zhu L, Jia W, et al. Validation and comparison of models to predict non-sentinel lymph node metastasis in breast cancer patients. Cancer Sci 2012;103:274-81. [Crossref] [PubMed]
- Friedman D, Gipponi M, Murelli F, et al. Predictive factors of non-sentinel lymph node involvement in patients with invasive breast cancer and sentinel node micrometastases. Anticancer Res 2013;33:4509-14. [PubMed]
- Chue KM, Yong WS, Thike AA, et al. Predicting the likelihood of additional lymph node metastasis in sentinel lymph node positive breast cancer: validation of the Memorial Sloan-Kettering Cancer Centre (MSKCC) nomogram. J Clin Pathol 2014;67:112-9. [Crossref] [PubMed]
- Dingemans SA, de Rooij PD, van der Vuurst de Vries RM, et al. Validation of Six Nomograms for Predicting Non-sentinel Lymph Node Metastases in a Dutch Breast Cancer Population. Ann Surg Oncol 2016;23:477-81. [Crossref] [PubMed]
- Zheng L, Liu F, Zhang S, et al. Nomograms for predicting the likelihood of non-sentinel lymph node metastases in breast cancer patients with a positive sentinel node biopsy. Medicine (Baltimore) 2019;98:e18522. [Crossref] [PubMed]
- Moosavi SA, Abdirad A, Omranipour R, et al. Clinicopathologic features predicting involvement of non- sentinel axillary lymph nodes in Iranian women with breast cancer. Asian Pac J Cancer Prev 2014;15:7049-54. [Crossref] [PubMed]
- Maimaitiaili A, Wu D, Liu Z, et al. Analysis of factors related to non-sentinel lymph node metastasis in 296 sentinel lymph node-positive Chinese breast cancer patients. Cancer Biol Med 2018;15:282-9. [Crossref] [PubMed]
- Sandoughdaran S, Malekzadeh M, Mohammad Esmaeil ME. Frequency and Predictors of Axillary Lymph Node Metastases in Iranian Women with Early Breast Cancer. Asian Pac J Cancer Prev 2018;19:1617-20. [PubMed]
- Castrucci W, Lannin D, Haffty BG, et al. Using nodal ratios to predict risk of regional recurrences in patients treated with breast conservation therapy with 4 or more positive lymph nodes. ISRN Surg 2011;2011:874814.


