
Analysis of the relationship between Ki-67 expression and chemotherapy and prognosis in advanced non-small cell lung cancer
Introduction
Lung cancer has become the leading cause of cancer-related death across China and worldwide (1,2). Approximately 80% of lung cancers are non-small cell lung cancer (NSCLC), which includes adenocarcinoma (ADC) and squamous cell carcinoma (SQCC). Lung cancer is still a major health threat faced by humans, and most patients are already in the late stages of the disease at the time of diagnosis (3). The objective response rate (ORR) of first-line chemotherapy for advanced NSCLC is 15–50% (4). According to previous studies, the median survival time ranges from 7.7 to 10.3 months, and the 1-year survival rate is approximately 30% (4,5). Ten years have passed since those studies were published, and cures for glandular cancer have changed because of the discovery of molecular markers that affect the disease’s biological behaviours (6,7). For example, epidermal growth factor receptor (EGFR) mutations serve as biomarkers for the use of tyrosine kinase inhibitors (TKIs), which have established TKIs as a first-line treatment for EGFR-positive NSCLC (8). The discovery of the anaplastic lymphoma kinase (ALK) rearrangement has laid the foundation for the development of TKIs that are aimed at ALK. Crizotinib has become the first-line treatment for patients with ALK rearrangement-positive tumours (9).
Although enormous progress has been made in molecular biology and targeted therapy, half of lung cancer patients have tumours that are devoid of specific molecular mutations. Moreover, although we can ascertain some molecular mechanisms, targeted therapy for some mutant genes is still impossible to achieve (e.g., RAS) (10). Currently, the platinum-based regime of third-generation cytotoxic chemotherapies is the standard treatment for patients lacking EGFR mutations or ALK rearrangement (4). For such patients, predictions of chemosensitivity have not involved specific biological markers (11). Ki-67 is a DNA-binding nucleoprotein that is expressed throughout the cell cycle but not in resting (G0) cells. It is a well-known potent biomarker and has great predictive value in cancers such as small lung cell cancer (12) and breast cancer (13). Therefore, in multiple solid tumour types, the Ki-67 index has directly influenced clinical treatment decision-making and prognosis. However, use of the Ki-67 index has led to heated controversies in various studies regarding its ability to predict curative effects and prognosis of patients with advanced NSCLC. To further understand the clinical significance of Ki-67 in advanced NSCLC, we retrospectively analysed the relationship between Ki-67 index and the ORR and PFS based on data of 112 advanced NSCLC patients collected by Anhui Chest Hospital from 2014 to 2017.
Methods
Patient characteristics and tumour samples
All selected cases were advanced NSCLC specimens from patients who were hospitalized from 2014 to 2017. The clinicopathological characteristics examined included sex, age, weight, body mass index, Karnofsky status, stage, presence of distant metastasis, histologic type, chemotherapy, Ki-67 index, complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD), ORR and PFS. All tumour samples were obtained by bronchoscopy and were thus suitable for immunohistochemistry. All patients received four cycles of chemotherapy. Tumours were staged according to the Seventh Edition of the Union for International Cancer Control (U.I.C.C.)/American Joint Committee on Cancer Tumor, Node, Metastasis (TNM) staging system. After patients received two cycles of chemotherapy, all target lesions were imaged by our hospital’s chest CT scanner. The curative effect was assessed by Response Evaluation Criteria in Solid Tumors (RECIST version1.1) of the European Organization for Research and Treatment of Cancer (EORTC). This study was authorized by the Ethics Committee of Anhui Chest Hospital (No. K2020002), and all patients signed an informed consent.
Immunohistochemistry and Ki-67 analysis
Immunohistochemistry was performed using the streptavidin peroxidase (SP) method. Ki-67 antibody [anti-Ki-67 (30-9) rabbit monoclonal antibody, Ventana Medical Systems, Inc., USA] was purchased. Experimental procedures and operations were strictly performed according to reagent specification; the monoclonal antibodies against Ki-67 were diluted 1:100. The Ki-67 index was calculated as follows: 10 sections in each field were selected under 400× magnification, and 100 cells per field were counted until counts reached 1,000 cells; the percentage of positive expression in each section was then calculated. Next, we determined the percentage of positive expression in each section, and any value over 10% was identified as positive expression. In the negative control, we substituted PBS for the primary antibody.
Statistics
The values in each group were expressed as ; a comparison of the clinicopathological characteristics between the objective response group and the non-response group was performed by unpaired t-test, and related factors that affected the ORR of chemotherapy were analysed using a logistic regression model. A receiver operating characteristic (ROC) curve was constructed by plotting sensitivity vs. [100%-specificity (%)]. The ROC curves were used to evaluate the accuracy of the Ki-67 index in predicting ORR and to derive the corresponding best cut-off values. PFS was analysed for all possible cut-off values using the Kaplan-Meier method, with a log-rank test to probe for significance. A Cox proportional hazards regression model was used for the univariate and multivariate analyses of PFS. All tests were bilateral and were performed using SPSS 16.0 (IBM SPSS, Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad Software, Inc., USA). P values <0.05 were considered significant.
Results
Clinicopathological characteristics
The test cohort included 112 NSCLC specimens: 57 ADC (50.9%) and 55 SQCC (49.1%). The mean age of the patients was 61.3 years (range, 30–79 years), and 75 patients were male (67.0%) and 37 were female (33.0%). Thirty-three (29.5%) tumours were classified as stage IIIB and 79 (70.5%) were classified as stage IV. After two cycles of palliative chemotherapy with a platinum-containing regimen, the ORR was evaluated clinically (61.6%, 69/112) and included the following: 0 cases of CR (0%, 0/112), 69 cases of PR (61.6%, 69/112), 41 cases of SD (36.6%, 41/112), and 2 cases of PD (1.8%, 2/112). In the SQCC group, 38 subjects exhibited an objective response (remission rate was 69.1%, 38/55): 0 cases of CR (0%, 0/112), 38 cases of PR (69.1%, 38/55), 16 cases of SD (29.1%, 16/55), and 1 case of PD (1.8%, 1/55). In the ADC group, 31 subjects exhibited an objective response (remission rate was 54.4%, 31/57): 0 cases of CR (0%, 0/57), 31 cases of PR (54.4%, 31/57), 25 cases of SD (43.9%, 25/57), and 1 case of PD (1.8%, 1/57).
According to the ORR of the patients, we divided them into the CR+PR group and the SD+PD group and compared their pathological characteristics (Table 1). After comparing the two sets of data, we found significant differences in the Ki-67 index (P=0.000) and stage (P=0.025) between the two groups.
Table1
| Characteristic | CR+PR (n=69) | SD+PR (n=43) | P value |
|---|---|---|---|
| Ki-67 (%), mean ± SEM | 62.61±2.433 | 47.21±2.503 | 0.000 |
| Stage (IIIB/IV) | 28/41 | 9/34 | 0.025 |
| Pathology (squamous/adenocarcinoma) | 38/31 | 17/26 | 0.112 |
| Sex (male/female) | 42/27 | 32/11 | 0.179 |
| Age (years), mean ± SEM | 62.81±1.139 | 58.98±1.679 | 0.053 |
| Weight (kg), mean ± SEM | 64.54±0.814 | 66.19±0.947 | 0.202 |
| Body mass index, mean ± SEM | 23.23±0.222 | 22.99±0.241 | 0.495 |
| Karnofsky, mean ± SEM | 86.67±1.004 | 84.65±1.261 | 0.215 |
| Tumor location (left/right) | 32/37 | 23/20 | 0.242 |
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; SEM standard error of the mean.
Therefore, the univariate logistic regression and multivariate logistic regression analyses showed that the main factors that affected the ORR of chemotherapy in the two groups were Ki-67 index (B =−0.069, P=0.000) and stage (B =2.352, P=0.001) (Table 2). In addition, the Ki-67 index was positively correlated with the objective efficacy of chemotherapy.
Table 2
| Variables | ORR | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Weight | 0.926 | 0.743–1.154 | 0.492 | – | – | – | |
| Ki-67 | 0.959 | 0.937–0.981 | 0.000* | 0.934 | 0.906–0.962 | 0.000* | |
| Sex | 0.569 | 0.245–1.317 | 0.188 | – | – | – | |
| Age | 0.963 | 0.927–1.001 | 0.056 | – | – | – | |
| Stage | 2.58 | 1.072–6.207 | 0.000* | 10.41 | 2.973–34.417 | 0.001* | |
| Pathology | 1.875 | 0.865–4.064 | 0.111 | 2.517 | 0.995–6.366 | 0.051 | |
| Karnofsky | 0.970 | 0.925–1.018 | 0.214 | – | – | – | |
| Body mass index | 0.717 | 0.548–0.940 | 0.016* | – | – | – | |
| Tumor location | 0.636 | 0.293–1.357 | 0.238 | – | – | – | |
*, P<0.05. ORR, objective response rate; CI, confidence interval; HR, hazard ratio.
High Ki-67 index predicts significant response to chemotherapy
Based on the differences in Ki-67 expression, we applied a ROC curve analysis to predict how effective the Ki-67 index was when it was used to judge objective efficacy of chemotherapy, and we found that Ki-67 does accurately reflect this efficacy. In all subjects, the sensitivity was 93.02% [P<0.001, area under the curve (AUC) =0.7467, 95% confidence interval (CI): 0.6578–0.8356]. In the SQCC group, the sensitivity was 94.12% (P=0.0003, AUC =0.8065, 95% CI: 0.6922–0.9208). In the patients with ADC, the difference in the Ki-67 index between the CR+PR group and the SD+PD group was significant (P=0.0193, AUC =0.6810, 95% CI: 0.5360–0.8262) (Figure 1).
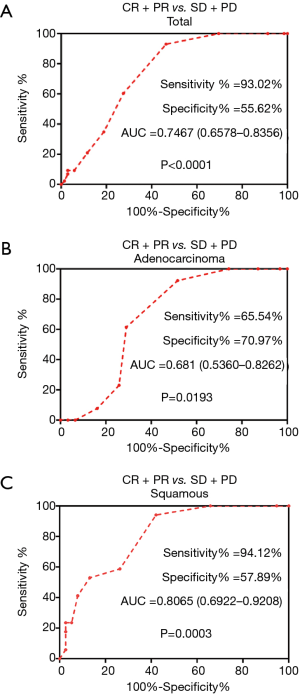
In the entire experimental group (both the SQCC and ADC groups), high Ki-67 expression was significantly associated with objective chemotherapy response. In the overall group and in the SQCC group, the best cut-off value for the Ki-67 index was 65% for the chemotherapy-effective group and the poor efficacy group. In the ADC group, the best cut-off value for the Ki-67 index was 55% for those with good chemotherapy efficacy and those with poor efficacy.
Low proliferation in SQCC and ADC is associated with better survival
These cut-off values indicated that the ORR of chemotherapy above the best cut-off value was better. All patients were divided into two groups according to the cut-off value of the Ki-67 index. We layered the cases and found that in all cases, the lower the Ki-67 index, the more evident the prediction for PFS (Figure 2).
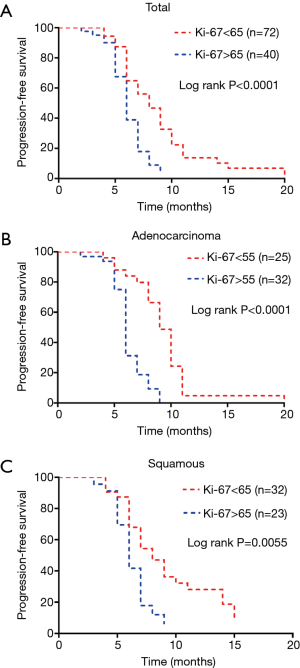
Next, we used a Cox proportional hazards regression model to perform univariate and multivariate analyses and found that Ki-67 index and stage together influenced PFS. Unlike the ORR, the Ki-67 index was a hazardous factor: the higher the Ki-67 index, the shorter the PFS time (B =0.32, P=0.000) (Table 3).
Table 3
| Variables | PFS | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Weight | 1.011 | 0.982–1.042 | 0.457 | – | – | – | |
| ORR | 1.127 | 0.745–1.705 | 0.572 | – | – | – | |
| Ki-67 | 1.034 | 1.021–1.048 | 0.000* | 1.027 | 1.013–1.041 | 0.000* | |
| Sex | 0.992 | 0.640–1.539 | 0.972 | – | – | – | |
| Age | 0.992 | 0.973–1.012 | 0.458 | – | – | – | |
| Stage | 3.226 | 1.934–5.382 | 0.000* | 2.405 | 1.400–4.130 | 0.001* | |
| Pathology | 1.164 | 0.774–1.752 | 0.466 | – | – | – | |
| Karnofsky | 0.992 | 0.967–1.017 | 0.535 | – | – | – | |
| Body mass index | 1.103 | 0.974–1.249 | 0.123 | – | – | – | |
| Tumor location | 1.328 | 0.881–2.002 | 0.175 | – | – | – | |
*, P<0.05. ORR, objective response rate; CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
Discussion
The Ki-67 gene is located on chromosome 10 and consists of two polypeptide chains whose molecular weights are 345 and 395 kDa, respectively. Ki-67 is expressed during the entire cell cycle and is associated with mitosis. Its expression reflects cell proliferation (14). In many tumours, the Ki-67 index, as an effective biomarker, has been used to predict curative treatment and has been used as a prognostic indicator in breast cancer (13) and prostate cancer (15). In recent years, a meta-analysis reported that high expression of Ki-67 was an adverse prognostic factor. However, the Ki-67 index still had uncertain value in predicting postoperative chemotherapy response in NSCLC stages I–III (16). In studies of NSCLC, in most cases, the Ki-67 index was a predictor of short disease-free survival (DFS) and overall survival (OS) (17,18). The Ki-67 index was also of some value in predicting the curative effects of neoadjuvant chemotherapy for NSCLC (19). Nevertheless, studies that have used the Ki-67 index to predict chemotherapeutic effects in advanced NSCLC are rare. In the current study, we also found that the Ki-67 index demonstrated independent positive relevance with the ORR of patients with advanced NSCLC. Here, the Ki-67 index was an adverse factor, rather than an independent factor, that influenced PFS.
One analysis from abroad of the clinical data of 1,150 NSCLC patients indicated that Ki-67 played a vital clinical role in NSCLC. However, that study found that Ki-67 index expression was clearly related to DFS and OS only in stage I–II ADC (17). When predicting the relationship between Ki-67 index and prognosis, most studies used the average or median value of the Ki-67 index in case stratification (20,21), while some adopted earlier Ki-67 thresholds in case stratification (22). However, in advanced NSCLC, the best and most widely applicable Ki-67 index threshold is not a definite tool that can be used to judge a patient’s prognosis. Since many individual studies have provided different results, the threshold of the Ki-67 index varies, and therefore, the application of the Ki-67 index in routine diagnosis and treatment of NSCLC has been hindered.
As a result, establishing the threshold used is crucial to assessing the ability of the Ki-67 index to predict the ORR of chemotherapy and prognosis. In this research, we first divided all the cases into two groups according to ORR and then compared the clinicopathologic characteristics. Finally, we performed univariate and multivariate regression analyses, thereby determining the optimal Ki-67 index threshold. Additionally, this research found that Ki-67 was not an independent factor that affected PFS, but together with tumour stage, it affected PFS time.
The clinical data we selected for this study were limited and could not be systematically retrospectively analysed for the clinical significance of Ki-67 expression in NSCLC. The efficacy of chemotherapy for advanced NSCLC is affected by many factors. Specifically, in the age of precision medicine, molecular targeted drugs have become the treatment of choice for advanced NSCLC patients. For those patients with driver gene mutations, such as those in EGFR, EGFR TKIs combined with chemotherapy have been a better choice (23). For such patients, further studies should be performed to understand how the Ki-67 index can be used to evaluate clinical curative effects.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.72). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was authorized by the Ethics Committee of Anhui Chest Hospital (No. K2020002), and all patients signed an informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Howlader N, Chen VW, Ries LA, et al. Overview of breast cancer collaborative stage data items--their definitions, quality, usage, and clinical implications: a review of SEER data for 2004-2010. Cancer 2014;120:3771-80. [Crossref] [PubMed]
- Goffin J, Lacchetti C, Ellis PM, et al. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol 2010;5:260-74. [Crossref] [PubMed]
- Janssen-Heijnen ML, van Erning FN, De Ruysscher DK, et al. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol 2015;26:902-7. [Crossref] [PubMed]
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Gandara DR, Li T, Lara PN Jr, et al. Algorithm for codevelopment of new drug-predictive biomarker combinations: accounting for inter- and intrapatient tumor heterogeneity. Clin Lung Cancer 2012;13:321-5. [Crossref] [PubMed]
- Olaussen KA, Postel-Vinay S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: a challenging landscape. Ann Oncol 2016;27:2004-16. [Crossref] [PubMed]
- Ishibashi N, Maebayashi T, Aizawa T, et al. Correlation between the Ki-67 proliferation index and response to radiation therapy in small cell lung cancer. Radiat Oncol 2017;12:16. [Crossref] [PubMed]
- Hadad SM, Jordan LB, Roy PG, et al. A prospective comparison of ER, PR, Ki67 and gene expression in paired sequential core biopsies of primary, untreated breast cancer. BMC Cancer 2016;16:745. [Crossref] [PubMed]
- Hoster E, Rosenwald A, Berger F, et al. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 2016;34:1386-94. [Crossref] [PubMed]
- Lobo J, Rodrigues Â, Antunes L, et al. High immunoexpression of Ki67, EZH2, and SMYD3 in diagnostic prostate biopsies independently predicts outcome in patients with prostate cancer. Urol Oncol 2018;36:161.e7-161.e17. [Crossref] [PubMed]
- Jakobsen JN, Sørensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer 2013;79:1-7. [Crossref] [PubMed]
- Warth A, Cortis J, Soltermann A, et al. Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer 2014;111:1222-9. [Crossref] [PubMed]
- Ahn HK, Jung M, Ha SY, et al. Clinical significance of Ki-67 and p53 expression in curatively resected non-small cell lung cancer. Tumour Biol 2014;35:5735-40. [Crossref] [PubMed]
- Hong X, Yang Z, Wang M, et al. Reduced decorin expression in the tumor stroma correlates with tumor proliferation and predicts poor prognosis in patients with I-IIIA non-small cell lung cancer. Tumour Biol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Cardona AF, Rojas L, Wills B, et al. Pemetrexed/Carboplatin/Bevacizumab followed by Maintenance Pemetrexed/Bevacizumab in Hispanic Patients with Non-Squamous Non-Small Cell Lung Cancer: Outcomes according to Thymidylate Synthase Expression. PLoS One 2016;11:e0154293. [Crossref] [PubMed]
- Glatzel-Plucinska N, Piotrowska A, Grzegrzolka J, et al. SATB1 Level Correlates with Ki-67 Expression and Is a Positive Prognostic Factor in Non-small Cell Lung Carcinoma. Anticancer Res 2018;38:723-36. [PubMed]
- Berghoff AS, Ilhan-Mutlu A, Wohrer A, et al. Prognostic significance of Ki67 proliferation index, HIF1 alpha index and microvascular density in patients with non-small cell lung cancer brain metastases. Strahlenther Onkol 2014;190:676-85. [Crossref] [PubMed]
- Wen M, Xia J, Sun Y, et al. Combination of EGFR-TKIs with chemotherapy versus chemotherapy or EGFR-TKIs alone in advanced NSCLC patients with EGFR mutation. Biologics 2018;12:183-90. [PubMed]


