
Drug-eluting beads-transcatheter arterial chemoembolization with or without iodine-125 treatment is effective and tolerable in treating advanced non-small cell lung cancer patients: a pilot study
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer death which accounts for 11.6% of total cancer cases and 18.4% of total cancer deaths all around the world (1,2). According to a recent cancer statistic report, there are 2,093,876 new cases and 1,761,007 lung cancer deaths in 2018 (1). Non-small cell lung cancer (NSCLC) is the most prevalent subtype of lung cancer that approximately 85% of lung cancer cases are NSCLC patients (3). Among the various alternative treatments for NSCLC, surgery is mainly used to treat early-staged NSCLC patients, whereas most NSCLC patients are diagnosed at advanced stage (3,4). Radiotherapy [such as iodine-125 (125I) seed implantation] exhibits favorable treatment efficacy in majority of patients, whereas it is mainly served as an adjuvant therapy or palliative treatment due to the high recurrence rate (5). Targeted therapies (such as epidermal growth factor receptor tyrosine kinase inhibitors) and immunotherapies (such as anti–programmed death 1 antibody) provide great survival benefits for advanced NSCLC patients (6,7). However, there are still some patients who have no chance to receive these treatments partly due to lacking mutations, high cost or drug resistance (8). As for platinum-based systemic chemotherapy, it is commonly used as a palliative treatment which might decrease NSCLC patients’ quality of life and bring in psychological disorders due to its systemic adverse events (AEs) (9,10). Therefore, exploring additional and effective treatment strategies is still critical to improving the survival of NSCLC patients.
Drug-eluting beads-transcatheter arterial chemoembolization (DEB-TACE) is a locoregional treatment strategy, and the microspheres not only delivery chemotherapeutic drugs to tumor tissues long-lastingly via the feeding arteries, but also block blood supply of tumor tissues, which then cause tumor ischemia and necrosis (11,12). Accumulating evidences have illustrated that DEB-TACE is an effective method to not only improve survival, but also decrease fatal AEs in unresectable liver cancer patients, whereas there is still limited information regarding the efficacy and safety of DEB-TACE in other cancer patients (13,14). As for lung cancer, one previous study enrolls 17 metastatic lung cancer patients who are about to receive DEB-TACE plus radiofrequency (RF) ablation, and reveals that the DEB-TACE plus RF ablation is feasible, effective and safe in these unresectable lung cancer patients (15). However, that previous study focuses on the metastatic lung cancer patients, and the sample size is relatively small. More importantly, there is no more study reporting the application of DEB-TACE in treating advanced NSCLC patients. Therefore, we conducted the current study to explore the efficacy and safety of DEB-TACE with or without 125I seed implantation in treating advanced NSCLC patients.
Methods
Patients
Twenty-five advanced NSCLC patients underwent DEB-TACE or DEB-TACE combined with 125I seed implantation in Lishui Central Hospital between January 2016 and May 2018 were consecutively recruited in this study. The inclusion criteria were as follows: (I) diagnosed as NSCLC confirmed by pathology examination; (II) TNM stage III, which was assessed according to the Union for International Cancer Control (UICC) system (7th edition); (III) age above 18 years; (IV) underwent DEB-TACE or DEB-TACE combined with 125I seed implantation therapy; (V) Eastern Cooperative Oncology Group (ECOG) score ≤2. Patients were excluded if they had the following conditions: (I) coagulation dysfunction; (II) allergic to chemotherapy agents used in the TACE therapy; (III) severe infection, serious heart, kidney or liver dysfunction; (IV) life expectancy less than 12 months; (V) history of TACE or 125I seed implantation treatment; (VI) pregnant or lactating women.
Ethics statement
The current study protocol was approved by Ethics Committee of Lishui Central Hospital, and the study was performed according to the Declaration of Helsinki and Good Clinical Practice guidelines as defined by the International Conference on Harmonisation. Written informed consents were collected from all patients or their family members before enrollment.
Baseline data collection
Baseline characteristics of patients were collected after enrollment, including age, gender, history of smoke, tumor location, tumor size, histologic type, pathological differentiation, ECOG score and history of treatment. Besides, following baseline data were also collected: clinical symptoms (including cough, hemoptysis, chest distress, chest pain and dyspnea), hematological indexes [including white blood cell (WBC), red blood cell (RBC), absolute neutrophil count (ANC), hemoglobin (Hb) and platelet (PLT)] and tumor markers [including carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), 2-phospho-D-glycerate hydrolase (NSE), squamous cell carcinoma antigen (SCCAg), cytokeratin 19 fragment (Cyfra21-1)].
Treatment
After the eligibility of DEB-ATCE therapy was confirmed, patients received the DEB-TACE voluntarily, and then the response to DEB-TACE therapy was evaluated at 1 month post the treatment. For patients with incompletely necrotic tumors, 125I seed implantation was administered if they were willing to receive, and others who were unwilling to receiving the 125I seed implantation, systemic chemotherapy or radiotherapy was given according to the disease status and personal willingness.
DEB-TACE procedure
Chest computed tomography (CT) plain scan and enhanced scan were performed to determine the size and blood supply of the tumors, and to exclude the contraindication of vascular intervention before the operation. The patient was lying on the digital subtraction angiography (DSA) operating table with routine disinfection of bilateral inguinal skin. Under local anesthesia by 5 mL 2% lidocaine, the femoral artery was percutaneously punctured with Seldinger technique using 5F catheter sheath. Subsequently, 5F Cobra catheter (COOK, Bloomington, Indiana, USA) was selectively inserted into bronchial artery. Then bronchial arteriography was performed to detect the supplying artery of tumor using 300 mg/mL iohexol at an injection rate of 1–2 mL/s (total volume was 3 to 8 mL). If the supplying artery of tumor was found being in common trunk with spinal artery, esophageal artery or intercostal artery, Terumo Progreat 3F microcatheter (Terumo Corporation, Shibuya-ku, Tokyo, Japan) was superselectively placed in the tumor supplying artery by avoiding spinal artery, esophageal artery and intercostal artery. Next, 300–500 µm CalliSpheres® microspheres (CSM) (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) loaded with 1.0 g gemcitabine was injected at the speed of 1 mL/min into the tumor supplying artery under perspective monitoring and followed by 60 mg (30 mg/m2) cisplatin infusion, with an average infusion dose of 53.2±7.1 mg. The embolization was stopped when the tumor staining was disappeared. After embolization, angiography was conducted again to detect whether there was extravasation of contrast medium and incomplete embolization of tumor artery. When procedures of DEB-TACE were finished, the microcatheter was pulled out, and the wound was pressed for hemostasis and then bandaged.
125I seed implantation procedure
In the present study, 125I seed with radiation activity of 0.7–0.9 mCi was provided by Seeds Biological Pharmacy (Tianjin) Ltd. (Seeds Biological Pharmacy (Tianjin) Ltd., Tianjin, China), which was implanted using HGGR-3000 Radioactive Seed Treatment-Planning System (TPS) (Zhuhai Hokai Medical Instruments Co., Ltd., Zhuhai, Guangdong, China). Before seed implantation, patients’ clinical data were input into TPS to calculate the number, radiation activity and location of 125I seed. And CT scan was performed to determine the location, direction and depth of the needle insertion during operation. With 2% lidocaine local anesthesia, patients were required to hold breath, then an 18-gauge needle was gradually inserted into the tumor. After confirming the puncture needle in right position by CT scan, the turntable seed implantation gun was used to evenly implant 125I seed into the tumor at an interval of 0.5–1.0 cm, layer by layer. For tumors with residual thickness less than 1.0 cm, the 125I seed was evenly implanted with a 0.5 cm interval centered on the largest layer of the tumor. If the tumor was large, seed implantation was carried out by more times. The median number of implanted 125I seed was 49 (range, 28–70), and the median matched peripheral dose (MPD) was 89.31 Gy (range, 72.63–105.99 Gy). CT scan was performed immediately after seed implantation to confirm the implantation status of seed and detect whether complications (such as pneumothorax and hemothorax) occurred. After the operation, the needle was pulled out, and the wound was bandaged under pressure.
Assessments
Dynamic enhanced CT scan, clinical symptom examination and hematological examination were performed at 1 month after DEB-TACE and 125I seed implantation respectively. Tumor response to the DEB-TACE therapy and 125I seed implantation was assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), which was classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Moreover, objective response rate (ORR) was defined as CR+PR, and disease control rate (DCR) was defined as CR + PR + SD. Besides, adverse events occurred at 1 week after DEB-TACE and 125I seed implantation were also recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 respectively, and were categorized as pain, fever, nausea, vomit and others (16).
Follow up
In the first 3 months after treatment, all patients were followed up every month and then every 3 months until death or last follow-up (2018/10/28), with a median follow-up time of 9.1 months. Overall survival (OS) was calculated from the date of DEB-TACE therapy to the date of death or last follow-up.
Statistical analysis
Among enrolled 25 patients, there was 1 patient missing data of treatment response, therefore, a total of 24 patients were included in the analysis of response treatment; in addition, among 17 patients who underwent DEB-TACE combined with 125I seed implantation, 2 patients were excluded from the analysis of clinical symptoms, hematological indexes, tumor markers and adverse events after 125I seed implantation due to missing data. And all 25 patients were included in the survival analysis without missing data. For normally or approximatively normal distributed continuous variable, it was presented as mean value ± standard deviation; as for skewed or unknown distributed continuous variable, it was expressed as median (25th–75th quantiles), and the comparison between prior treatment and post treatment was determined by Wilcoxon signed rank test. Both ordered and unordered variable categorical variables were presented as count (percentage), and the comparison of ordered categorical variable between prior treatment and post treatment was determined using Wilcoxon signed rank test. OS was demonstrated by use of Kaplan-Meier method and comparison between subgroups was determined by log-rank test. SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for statistical data processing, and GraphPad Prism 6.01 software (GraphPad Software Inc., San Diego, CA, USA) was used for figures making. P value <0.05 was considered significant.
Results
Characteristics in advanced NSCLC patients
In totally 25 NSCLC patients, there were 19 male and 6 female, and the mean age was 67.3±9.8 years. The number of patients with smoking history was 13 (52.0%) and the mean tumor size was 6.0±2.5 cm. Meanwhile, 10 (40.0%) patients were squamous cell carcinoma, 12 (48.0%) patients were adenocarcinoma and 3 (12.0%) patients were mixed carcinoma. And the number of patients with ECOG score 0, 1, and 2 was 10 (40.0%), 14 (56.0%) and 1 (4.0%), respectively. Besides, 17 (68.0%) patients also received combined 125I seed implantation after DEB-TACE treatment, and the interval between the two treatments was 38.6±29.5 days. Other characteristics of NSCLC patients were listed in Table 1.
Table 1
| Characteristics | Values (total n=25) |
|---|---|
| Age (years) | 67.3±9.8 |
| Gender (male/female) | 19/6 |
| History of smoke, n (%) | 13 (52.0) |
| Tumor location, n (%) | |
| Left | 13 (52.0) |
| Right | 12 (48.0) |
| Tumor size (cm) | 6.0±2.5 |
| Histologic type, n (%) | |
| Squamous cell carcinoma | 10 (40.0) |
| Adenocarcinoma | 12 (48.0) |
| Mixed carcinoma | 3 (12.0) |
| Pathological differentiation, n (%) | |
| Well | 0 (0.0) |
| Moderate | 2 (8.0) |
| Poor | 23 (92.0) |
| ECOG score, n (%) | |
| 0 | 10 (40.0) |
| 1 | 14 (56.0) |
| 2 | 1 (4.0) |
| History of treatment, n (%) | |
| Interventional therapy | 1 (4.0) |
| Systematic chemotherapy | 19 (76.0) |
| Targeted therapy | 23 (92.0) |
| Combined with 125I seed implantation, n (%) | 17 (68.0) |
| The interval between DEB-TACE and 125I seed implantation (days) | 38.6±29.5 |
Data were presented as mean value ± standard deviation or count (percentage). ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small-cell lung cancer; 125I, iodine-125.
Treatment response rates in advanced NSCLC patients
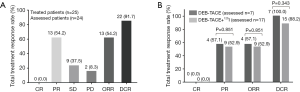
Treatment response was assessed in 24 NSCLC patients, among whom 7 patients received DEB-TACE and 17 patients received DEB-TACE plus 125I seed implantation. The number of patients achieved CR, PR, SD and PD were 0 (0.0%), 13 (54.2%), 9 (37.5%) and 2 (8.3%) respectively at 1 month after DEB-TACE or DEB-TACE plus 125I seed implantation, and the ORR and DCR were 13 (54.2%) and 22 (91.7%) (Figure 1A). For patients received DEB-TACE treatment, the CR, PR, ORR and DCR were 0 (0.0%), 4 (57.1%), 4 (57.1%) and 7 (100.0%) respectively at 1 month after treatment; for patients received DEB-TACE plus 125I seed implantation, the CR, PR, ORR and DCR were 0 (0.0%), 9 (52.9%), 9 (52.9%) and 15 (88.2%) respectively at 1 month after treatment; no difference of CR, PR, ORR or DCR was discovered between the two groups (all P>0.05) (Figure 1B).
OS and factors affecting OS in advanced NSCLC patients
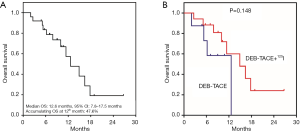
The median OS was 12.6 (95% CI: 7.8–17.5) months and the accumulating OS at 12th month was 47.6% in NSCLC patients (Figure 2A), besides, no difference of OS was found between patients receiving DEB-TACE and patients receiving DEB-TACE plus 125I seed implantation (P=0.148) (Figure 2B). In addition, we further investigated the correlation of baseline characteristics with OS in these NSCLC patients, and found that NSCLC patients with tumor size ≤5 cm presented with higher OS compared with patients with tumor size >5 cm (P=0.023) (Table S1). However, no association of age (P=0.828), gender (P=0.652), history of smoke (P=0.906), tumor location (P=0.461), histologic type (P=0.051), pathological differentiation (P=0.146), ECOG score (P=0.094), previous chemotherapy (P=0.224) or previous targeted therapy (P=0.453) with OS was observed in NSCLC patients.
Clinical symptoms before DEB-TACE treatment and at 1 month after DEB-TACE treatment in advanced NSCLC patients
The chest distress grade (P=0.013) and dyspnea grade (P=0.025) were decreased at 1 month after DEB-TACE treatment than that before treatment. However, no difference of cough grade (P=0.308), hemoptysis grade (P=0.564) or chest pain grade (P=1.000) was observed in NSCLC patients before DEB-TACE and at 1 month after DEB-TACE treatment (Table 2).
Table 2
| Clinical symptoms | Before DEB-TACE (n=25) | One month after DEB-TACE (n=25) | P |
|---|---|---|---|
| Cough grade, n (%) | 0.308 | ||
| No | 10 (40.0) | 8 (32.0) | |
| Mild | 9 (36.0) | 16 (64.0) | |
| Moderate | 4 (16.0) | 1 (4.0) | |
| Severe | 2 (8.0) | 0 (0.0) | |
| Hemoptysis grade, n (%) | 0.564 | ||
| No | 22 (88.0) | 23 (92.0) | |
| Mild | 3 (12.0) | 2 (8.0) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Chest distress grade, n (%) | 0.013 | ||
| No | 15 (60.0) | 22 (88.0) | |
| Mild | 7 (28.0) | 3 (12.0) | |
| Moderate | 3 (12.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Chest pain grade, n (%) | 1.000 | ||
| No | 23 (92.0) | 22 (88.0) | |
| Mild | 1 (4.0) | 3 (12.0) | |
| Moderate | 1 (4.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Dyspnea grade, n (%) | 0.025 | ||
| No | 19 (76.0) | 24 (94.0) | |
| Mild | 6 (24.0) | 1 (6.0) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) |
Data were presented as count (percentage). Comparisons were determined by Wilcoxon signed rank test. P<0.05 was considered significant. DEB-TACE, drug-eluting beads-transcatheter arterial chemoembolization.
Hematological indexes and tumor markers before DEB-TACE treatment and at 1 month after DEB-TACE treatment in advanced NSCLC patients
At 1 month after DEB-TACE treatment, median values of RBC (P=0.044), Hb (P=0.007) and PLT (P=0.042) were declined, while median value of ANC (P<0.001) was elevated compared to that before DEB-TACE treatment (Table 3). Whereas there was no difference in other hematological indexes or tumor markers in NSCLC patients before DEB-TACE and at 1 month after treatment (all P>0.05).
Table 3
| Items | Before DEB-TACE (n=25) | One month after DEB-TACE (n=25) | P |
|---|---|---|---|
| WBC (×109/L) | 6.7 (5.5–7.8) | 6.9 (6.0–9.0) | 0.095 |
| RBC (×1012/L) | 3.9 (3.7–4.2) | 3.6 (3.5–4.2) | 0.044 |
| ANC (%) | 66.9 (60.0–73.1) | 77.5 (72.3–87.2) | <0.001 |
| Hb (g/L) | 122.0 (112.0–132.0) | 117.0 (106.0–125.5) | 0.007 |
| PLT (×109/L) | 248.0 (181.0–317.0) | 208.0 (166.5–296.0) | 0.042 |
| CEA (ng/mL) | 3.2 (2.2–10.0) | 3.0 (1.6–12.2) | 0.502 |
| CA125 (U/mL) | 41.7 (14.8–201.1) | 83.4 (18.9–182.2) | 0.859 |
| NSE (U/mL) | 18.9 (15.2–35.2) | 13.4 (11.4–55.1) | 1.000 |
| SCCAg (ng/mL) | 1.2 (0.8–4.4) | 0.9 (0.5–7.5) | 0.753 |
| Cyfra21-1 (ng/mL) | 6.0 (3.9–12.2) | 5.7 (3.3–22.3) | 0.594 |
Data were presented as median (25th-75th quantiles). Comparisons were determined by Wilcoxon signed rank test. P<0.05 was considered significant. DEB-TACE, drug-eluting beads-transcatheter arterial chemoembolization; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; CEA, carcinoembryonic antigen; CA125, Carbohydrate antigen 125; NSE, 2-phospho-D-glycerate hydrolase; SCCAg, squamous cell carcinoma antigen; Cyfra21-1, cytokeratin 19 fragment.
Adverse events within 1 week after DEB-TACE treatment in advanced NSCLC patients
As for adverse events by DEB-TACE operation, 3 (12.0%), 8 (32.0%), 1 (4.0%), 1 (4.0%) and 8 (32.0%) NSCLC patients occurred pain, fever, nausea, vomit and other adverse events respectively within 1 week after DEB-TACE treatment (Table 4).
Table 4
| Adverse events | Patients (n=25) |
|---|---|
| Pain, n (%) | 3 (12.0) |
| Fever, n (%) | 8 (32.0) |
| Nausea, n (%) | 1 (4.0) |
| Vomit, n (%) | 1 (4.0) |
| Others, n (%) | 8 (32.0) |
Data were presented as count (percentage). DEB-TACE, drug-eluting beads-transcatheter arterial chemoembolization.
Clinical symptoms before 125I seed implantation and at 1 month after 125I seed implantation in advanced NSCLC patients
There was no difference of cough grade (P=0.783), hemoptysis grade (P=0.317), chest distress grade (P=0.317), chest pain grade (P=1.000) or dyspnea grade (P=1.000) in NSCLC patients before 125I seed implantation and at 1 month after 125I seed implantation (Table 5).
Table 5
| Clinical symptoms | Before 125I seed implantation (assessed n=15) | One month after 125I seed implantation (assessed n=15) | P |
|---|---|---|---|
| Cough grade, n (%) | 0.783 | ||
| No | 8 (53.3) | 5 (33.3) | |
| Mild | 5 (33.3) | 9 (60.0) | |
| Moderate | 1 (6.7) | 1 (6.7) | |
| Severe | 1 (6.7) | 0 (0.0) | |
| Hemoptysis grade, n (%) | 0.317 | ||
| No | 15 (100.0) | 14 (93.3) | |
| Mild | 0 (0.0) | 1 (6.7) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Chest distress grade, n (%) | 0.317 | ||
| No | 11 (73.3) | 12 (80.0) | |
| Mild | 4 (26.7) | 3 (20.0) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Chest pain grade, n (%) | 1.000 | ||
| No | 13 (86.7) | 11 (73.3) | |
| Mild | 1 (6.7) | 4 (26.7) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 1 (6.7) | 0 (0.0) | |
| Dyspnea grade, n (%) | 1.000 | ||
| No | 15 (100.0) | 15 (100.0) | |
| Mild | 0 (0.0) | 0 (0.0) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) |
Data were presented as count (percentage). Comparisons were determined by Wilcoxon signed rank test. P<0.05 was considered significant. 125I, iodine-125.
Hematological indexes and tumor markers before 125I seed implantation and at 1 month after 125I seed implantation in advanced NSCLC patients
The values of WBC (P=0.085), RBC (P=0.859), ANC (P=0.086), Hb (P=0.859), PLT (P=0.139), CEA (P=0.593), CA125 (P=1.000), NSE (P=0.785), SCCAg (P=0.465) and Cyfra21-1 (P=1.000) were similar in NSCLC patients before 125I seed implantation and at 1 month after 125I seed implantation (Table 6).
Table 6
| Clinical symptoms | Before 125I seed implantation (assessed n=15) | One month after 125I seed implantation (assessed n=15) | P |
|---|---|---|---|
| WBC (×109/L) | 5.5 (4.4–7.3) | 6.7 (5.5–9.1) | 0.085 |
| RBC (×1012/L) | 3.7 (3.3–4.2) | 3.6 (3.4–4.0) | 0.859 |
| ANC (%) | 66.6 (57.9–73.2) | 72.2 (61.8–79.6) | 0.086 |
| Hb (g/L) | 111.5 (104.5–127.5) | 113.5 (105.8–123.8) | 0.859 |
| PLT (×109/L) | 225.0 (123.0–266.5) | 221.5 (130.8–245.0) | 0.139 |
| CEA (ng/mL) | 2.9 (1.8–4.0) | 8.6 (2.0–285.1) | 0.593 |
| CA125 (U/mL) | 19.8 (10.7–67.0) | 67.9 (16.8–119.7) | 1.000 |
| NSE (U/mL) | 16.7 (15.3–20.8) | 19.2 (15.4–21.5) | 0.785 |
| SCCAg (ng/mL) | 1.4 (0.9–4.9) | 1.1 (0.5–12.7) | 0.465 |
| Cyfra21-1 (ng/mL) | 5.2 (2.9–9.5) | 7.9 (2.5–13.2) | 1.000 |
Data were presented as median (25th–75th quantiles). Comparisons were determined by Wilcoxon signed rank test. P<0.05 was considered significant. 125I, iodine-125; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; CEA, carcinoembryonic antigen; CA125, Carbohydrate antigen 125; NSE, 2-phospho-D-glycerate hydrolase; SCCAg, squamous cell carcinoma antigen; Cyfra21-1, cytokeratin 19 fragment.
Adverse events within 1 week after 125I seed implantation in advanced NSCLC patients
4 (26.7%) patients occurred pain and 8 (53.3%) patients occurred other adverse advents within 1 week after 125I seed implantation, while no patient occurred fever, nausea or vomit (Table 7).
Table 7
| Adverse events | Patients (assessed n=15) |
|---|---|
| Pain, n (%) | 4 (26.7) |
| Fever, n (%) | 0 (0.0) |
| Nausea, n (%) | 0 (0.0) |
| Vomit, n (%) | 0 (0.0) |
| Others, n (%) | 8 (53.3) |
Data were presented as count (percentage). 125I, iodine-125.
Discussion
Accumulating evidences reveal that chemoembolization is effective for the treatment of lung cancer (17,18). For instance, a prospective cohort study observes that 8 (34.8%) cancer patients with unresectable lung metastases achieve PR and 6 (26.1%) patients achieve SD after transpulmonary chemoembolization (the suspension of mitomycin and lipiodol is injected into the feeding artery, followed by embolization with microspheres) (19). In another prospective cohort study, 52 unresectable lung cancer patients receive transpulmonary chemoembolization, among whom 16 (30.8%) patients achieve PR, 11 (21.2%) patients achieve SD, and 25 (48.1%) patients achieve PD; and the median OS is 21.1 (95% CI: 4.2–38) months (20). These previous studies have identified the efficacy of chemoembolization in lung cancer treatment. DEB-TACE uses microspheres to act both as drug carriers and embolization agents, which not only release chemotherapeutic drugs to tumor tissues in a sustained manner, but also block blood supply of tumor (12,13). These features enable DEB-TACE to be more effective in treating liver cancer patients compared with other chemoembolization methods (21). As for the feasibility of DEB-TACE in lung cancer treatment, one previous study recruits 17 metastatic lung cancer patients who are about to receive DEB-TACE plus RF ablation, and discovers that DEB-TACE plus RF ablation is effective and safe in these patients (15). However, that previous study focuses on the metastatic lung cancer patients but not advanced NSCLC patients. In the current study, we discovered that NSCLC patients who received DEB-TACE or DEB-TACE plus 125I seed implantation achieved an ORR of 54.2% and a DCR of 91.7%, and the median OS was 12.6 (95% CI: 7.8–17.5) months, indicating the favorable treatment efficacy of DEB-TACE in NSCLC patients. The possible reasons for the good efficacy of DEB-TACE in treating advanced NSCLC patients were as follows: (I) the sustained release of chemotherapeutic drugs by microspheres enabled the long-lasting tumoricidal efficacy, thus NSCLC patients who were treated by DEB-TACE exhibited favorable response and OS; (II) the microspheres also embolized feeding artery completely, leading to tumor ischemia and necrosis, which enhanced the anti-tumor efficacy of chemotherapeutic drugs. Interestingly, we also observed that the treatment response and OS were similar after DEB-TACE or DEB-TACE plus 125I seed implantation in NSCLC patients. The possible reasons may be due to: (I) The sample size was relatively small, causing the relatively low statistics power; (II) a proportion of NSCLC patients presented with incompletely necrotic tumors after DEB-TACE treatment and they subsequently received systemic chemotherapy or radiotherapy rather than 125I seed implantation. These patients might achieve similar response and OS compared with patients treated with DEB-TACE plus 125I seed implantation. Hence, the response or OS was of no difference after DEB-TACE treatment or DEB-TACE plus 125I seed implantation in NSCLC patients. In addition, we used DEB-TACE loaded with gemcitabine combined with cisplatin in this study, which is not a consensus for NSCLC treatment, however, gemcitabine and cisplatin were both common therapeutics in NSCLC treatment. In addition, DEB-TACE loaded with gemcitabine in treating NSCLC is also reported in a previous study (22).
The clinical symptoms of common AEs must be treated properly to avoid other life-threatening complications in NSCLC patients. According to previous studies, chemoembolization facilitates the alleviation of NSCLC patients’ clinical symptoms (15,23). In addition, 7 (70%) patients present with pulmonary lesion shrinkage, revealing that chemoembolization alleviates clinical symptoms of lung cancer patients (23). In accordance with this previous study, we found that DEB-TACE eased the clinical manifestations in advanced NSCLC patients, such as the chest distress grade and the dyspnea grade. The possible explanations for the results might derive from the sustained release of chemotherapeutic drugs and the complete embolization of feeding artery by microspheres. We also observed that the symptoms remained unchanged after 125I seed implantation, which might be due to that: advanced NSCLC patients who received 125I seed implantation also received DEB-TACE treatment before 125I seed implantation, which meant that these patients presented with alleviated symptoms before 125I seed implantation. Thus, it was difficult to realize improvement of symptoms after 125I seed implantation. Besides, we performed DEB-TACE before 125I radiation to diminish the risk of bleeding and to enhance the necrotic effect on tumor in NSCLC patients.
In order to further investigate its safety, we evaluated the AEs of NSCLC patients after DEB-TACE treatment. It showed that the most common AEs were mild fever and pain, implying that DEB-TACE was tolerable in treating NSCLC patients. Our results were in line with a previous study, which shows that pleural effusion, chest pain and fever are common AEs after lung cancer patients being treated by DEB-TACE plus RF ablation, and no treatment-related lung function changes or treatment-related deaths are observed (15). In brief, the AEs of DEB-TACE was tolerable in advanced NSCLC patients.
There still remained some limitations in the present study. Firstly, our study was a single-center study with a relatively small sample size, hence further validation is needed in multi-central study with more NSCLC patients. Secondly, the comparison of efficacy and safety between DEB-TACE and other chemoembolization methods (such as conventional transcatheter arterial chemoembolization or transpulmonary chemoembolization) was not explored. Thirdly, the use of DEB-TACE from bronchial artery was not established, however, there have been studies illustrating that DEB-TACE was tolerable and effective in tumors supplied by bronchial arteries, including NSCLC (22,24,25). Fourthly, the grading of AEs was not performed in our study, however, the primary aim of ours was to assess the incidence of AEs; besides, the number of AEs was small and most of which were tolerable. Lastly, the feasibility of DEB-TACE in treating other types of lung cancer (such as small cell lung cancer) also needed to be investigated.
Conclusions
In conclusion, DEB-TACE with or without 125I seed implantation is effective and tolerable in treating advanced NSCLC patients, which provides a good option for advanced NSCLC treatment.
Table S1
| Items | Mean OS (95% CI) (months) | P value |
|---|---|---|
| Age, years | 0.828 | |
| <60 | 8.2 (4.7–11.8) | |
| ≥60 | 14.2 (10.4–18.1) | |
| Gender | 0.652 | |
| Female | 10.5 (6.9–14.0) | |
| Male | 14.8 (10.3–19.3) | |
| History of smoke | 0.906 | |
| No | 11.8 (8.1–15.5) | |
| Yes | 14.0 (9.2–18.7) | |
| Tumor location | 0.461 | |
| Left | 15.4 (9.6–21.2) | |
| Right | 11.6 (8.2–15.0) | |
| Tumor size, cm | 0.023 | |
| ≤5 | 19.0 (12.5–25.5) | |
| >5 | 10.4 (7.3–13.6) | |
| Histologic type | 0.051 | |
| Squamous cell carcinoma | 9.0 (5.6–12.4) | |
| Adenocarcinoma | 17.4 (11.5–23.4) | |
| Mixed carcinoma | 12.0 (0.7–23.4) | |
| Pathological differentiation | 0.146 | |
| Moderate | – | |
| Poor | – | |
| ECOG score | 0.094 | |
| 0 | 7.7 (5.5–10.0) | |
| 1/2 | 15.6 (11.4–19.7) | |
| Previous chemotherapy | 0.224 | |
| No | 16.3 (10.4–22.3) | |
| Yes | 11.0 (6.3–15.6) | |
| Previous targeted therapy | 0.453 | |
| No | 15.2 (10.9–19.4) | |
| Yes | 12.0 (10.9–13.2) |
Correlation was determined by Log-rank test. OS, overall survival; ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small cell lung cancer; 125I, iodine-125.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The current study protocol was approved by Ethics Committee of Lishui Central Hospital. Written informed consents were collected from all patients or their family members before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Yoda S, Dagogo-Jack I, Hata AN. Targeting oncogenic drivers in lung cancer: Recent progress, current challenges and future opportunities. Pharmacol Ther 2019;193:20-30. [Crossref] [PubMed]
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. [Crossref] [PubMed]
- Mun M, Nakao M, Matsuura Y, et al. Video-assisted thoracoscopic surgery lobectomy for non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2018;66:626-31. [Crossref] [PubMed]
- Wink KCJ, van Baardwijk A, Troost EGC, et al. Nodal recurrence after stereotactic body radiotherapy for early stage non-small cell lung cancer: Incidence and proposed risk factors. Cancer Treat Rev 2017;56:8-15. [Crossref] [PubMed]
- Shroff GS, Benveniste MF, de Groot PM, et al. Imaging on Lung Cancer and Treatment with Targeted Therapy. Semin Ultrasound CT MR 2018;39:308-13. [Crossref] [PubMed]
- Liang H, Liu X, Wang M. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther 2018;11:6189-96. [Crossref] [PubMed]
- Shash E, Peccatori FA, Azim HA Jr. Optimizing the use of epidermal growth factor receptor inhibitors in advanced non-small-lung cancer (NSCLC). J Thorac Dis 2011;3:57-64. [PubMed]
- Zhang R, Jia M, Xu Y, et al. An ERCC4 regulatory variant predicts grade-3 or -4 toxicities in patients with advanced non-small cell lung cancer treated by platinum-based therapy. Int J Cancer 2018;142:1218-29. [Crossref] [PubMed]
- Li YQ, Zhang XY, Chen J, et al. ATP7B rs9535826 is associated with gastrointestinal toxicity of platinum-based chemotherapy in nonsmall cell lung cancer patients. J Cancer Res Ther 2018;14:881-6. [Crossref] [PubMed]
- Lewis AL, Dreher MR. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J Control Release 2012;161:338-50. [Crossref] [PubMed]
- Lewis AL, Holden RR. DC Bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Deliv 2011;8:153-69. [Crossref] [PubMed]
- Greco G, Cascella T, Facciorusso A, et al. Transarterial chemoembolization using 40 microm drug eluting beads for hepatocellular carcinoma. World J Radiol 2017;9:245-52. [Crossref] [PubMed]
- Yu CY, Ou HY, Weng CC, et al. Drug-Eluting Bead Transarterial Chemoembolization as Bridge Therapy for Hepatocellular Carcinoma Before Living-Donor Liver Transplantation. Transplant Proc 2016;48:1045-8. [Crossref] [PubMed]
- Gadaleta CD, Solbiati L, Mattioli V, et al. Unresectable lung malignancy: combination therapy with segmental pulmonary arterial chemoembolization with drug-eluting microspheres and radiofrequency ablation in 17 patients. Radiology 2013;267:627-37. [Crossref] [PubMed]
- Liu YJ, Zhu GP, Guan XY. Comparison of the NCI-CTCAE version 4.0 and version 3.0 in assessing chemoradiation-induced oral mucositis for locally advanced nasopharyngeal carcinoma. Oral Oncol 2012;48:554-9. [Crossref] [PubMed]
- Vogl TJ, Shafinaderi M, Zangos S, et al. Regional chemotherapy of the lung: transpulmonary chemoembolization in malignant lung tumors. Semin Intervent Radiol 2013;30:176-84. [Crossref] [PubMed]
- Vogl TJ, Nour-Eldin NE, Naguib NN, et al. Feasibility of assessing pulmonary blood volume using C-arm CT during transpulmonary chemoperfusion and chemoembolization in primary and secondary lung tumours. Br J Radiol 2016;89:20150244. [Crossref] [PubMed]
- Vogl TJ, Wetter A, Lindemayr S, et al. Treatment of unresectable lung metastases with transpulmonary chemoembolization: preliminary experience. Radiology 2005;234:917-22. [Crossref] [PubMed]
- Vogl TJ, Lehnert T, Zangos S, et al. Transpulmonary chemoembolization (TPCE) as a treatment for unresectable lung metastases. Eur Radiol 2008;18:2449-55. [Crossref] [PubMed]
- Makary MS, Kapke J, Yildiz V, et al. Conventional versus Drug-Eluting Bead Transarterial Chemoembolization for Neuroendocrine Tumor Liver Metastases. J Vasc Interv Radiol 2016;27:1298-304. [Crossref] [PubMed]
- Bie Z, Li Y, Li B, et al. The efficacy of drug-eluting beads bronchial arterial chemoembolization loaded with gemcitabine for treatment of non-small cell lung cancer. Thorac Cancer 2019;10:1770-8. [Crossref] [PubMed]
- Seki A, Shimono C. Transarterial chemoembolization for management of hemoptysis: initial experience in advanced primary lung cancer patients. Jpn J Radiol 2017;35:495-504. [Crossref] [PubMed]
- Song J, Chen W, Zhu X, et al. Short-term efficacy, safety, and cost-effectiveness of transarterial chemoembolization with drug-eluting beads versus synchronous radiochemotherapy for cervical cancer. Int J Gynaecol Obstet 2019;147:29-35. [Crossref] [PubMed]
- Ni JY, Sun HL, Chen YT, et al. Drug-eluting bead transarterial chemoembolization in the treatment for unresectable soft tissue sarcoma refractory to systemic chemotherapy: a preliminary evaluation of efficacy and safety. J Cancer Res Clin Oncol 2018;144:157-63. [Crossref] [PubMed]


