Accurate short tandem repeat analysis for confirmation of rare gestational adrenal choriocarcinoma: a case report
Introduction
Choriocarcinoma is a vascular aggressive tumor. There are two types of choriocarcinoma, gestational (secondary) choriocarcinoma (GC) and nongestational (primary) choriocarcinoma (NGC). It is important for clinicians to distinguish between these two types of tumor because of their different treatment and prognosis (1-6). Up till now, most of the literatures have mainly used two methods for auxiliary diagnosis. First is the time between diagnosed choriocarcinoma and last normal or abnormal pregnancy. The patient will be suspected with primary choriocarcinoma if the time period is more than 3 years (7). The diagnosis will be confirmed if the patient has menopause or is nulliparous (4,6). Second is the ultrasound of the pelvic cavity (8-11). If any mass is found in both pelvic cavity and extragonadal area in the ultrasound, the diagnosis is likely to be secondary choriocarcinoma (9). However, these methods mislead doctors sometimes because both of the detections can’t give an accurate diagnosis, which could lead to false treatment (6,7,12). According to what we have studied, some researchers used STR as a method to identify nongestational choriocarcinoma (5,6,13,14). However, they are all about choriocarcinoma in genital system. Up till now there hasn’t been a case in which a gestational adrenal choriocarcinoma using STR was reported.
Choriocarcinoma is a malignant carcinoma that easily spreads to the lung, the brain, and the liver (11). Adrenal choriocarcinoma is extremely rare (7,15). Herein, we describe a case of a 29-year-old woman who was diagnosed with adrenal choriocarcinoma. If the tumor was GC, the chromosomes of tumor cells should be similar to paternal chromosomes (7). To find the origin of the tumor, short tandem repeat (STR) (13) and pathologic analysis were used. Also, some related literatures were reviewed and concluded to differentiate between GC and NGC.
Case presentation
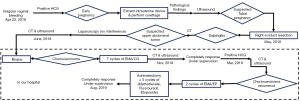
A 29-year-old woman visited several hospitals due to her unusual experience of seeking medical treatment from April 2018. Her medical history was not remarkable. She had her menophania at the age of 16. The period was 30–40 days and lasted for 6–7 days on average. Her menstruation seemed normal. There was no blood clot or dysmenorrhea during periods. She got married at the age of 20 and had 2 male babies (gravida 4 para 2) in 2009 and 2012, respectively. She had two induced abortions in 2015 and 2016 due to early pregnancy. Family history was not remarkable.
She was examined in a local hospital due to amenorrhea and irregular vaginal bleeding on April 23, 2018. Her serum human chorionic gonadotropin (β-hCG) was 634.2 mIU/mL. The intrauterine device was extracted and curettage was performed for the diagnosis of early pregnancy. The pathologic result showed various blood clots and a small amount of hypertrophic endometrium. The serum β-hCG increased to 3,295 mIU/mL after curettage. An ultrasound of the pelvic cavity was scheduled. There was a 1.6×0.8 cm mix-echoic mass on the right adnexa. She was suspected with tubal pregnancy. Thus, in early May 2018, a laparoscopic right-oviduct resection and a ligation of the left fallopian tube were performed. Intraoperative specimen was taken for pathologic research and the result was salpingitis.
After laparoscopy, her β-hCG increased from 2,381 to 4,073 mIU/mL and finally reached 11,204 mIU/mL in early June 2018. A computed tomography (CT) scan of her chest and the whole abdomen showed exudation and bilateral multiple lung nodules with little effusion in bilateral chest cavity. A mass in the left adrenal gland measuring 11×10 cm was suspected to be tumor. The laparoscopy was performed again in another local hospital. During the surgery, the mass was explored on the left adrenal gland (Figure 1A) with a large number of blood vessels. The doctors removed nothing during the operation. The patient was then transmitted to the Urologic Surgery Department of the First Affiliated Hospital of Xi’an Jiaotong University.
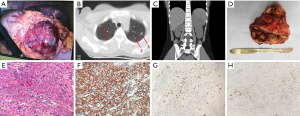
When admitted, her β-hCG was 8,182 mIU/mL. The ultrasound of the pelvis showed a slightly large uterus with rich blood flow on the interior wall. A CT scan of the cephalon, chest, and entire abdomen demonstrated multiple bilateral small nodules of the lung (five nodules in the left lung and four nodules in the right lung) (Figure 1B). A 7.6×10.3×11.0 cm mass was present in the left adrenal gland with an uneven inner density and a complete capsule (Figure 1C). A fine-needle biopsy was performed on the mass of the left adrenal gland, and afterward the pathologic diagnosis of choriocarcinoma was done. Based on Federation International of Gynecology and Obstetrics prognosis rating, the patient was diagnosed with adrenal choriocarcinoma (IV stage and score 13). Seven cycles of Etoposide (100 mg/m2), Actinomycin D (0.5 mg), Methotrexate (100 mg/m2), Vincristine (1.0 mg/m2), and Cyclophosphamide (600 mg/m2) were administrated to her. The serum β-hCG gradually decreased to 0.6 mIU/mL in November 2018. Meanwhile, CT scan and ultrasound were also performed alternatively. The size of the tumor decreased slightly, and the amount of the vessels declined. She was then followed-up closely in the next 3 months.
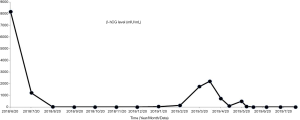
However, in March 2019, the patient demonstrated a gradual increase in β-hCG. Both CT scan and ultrasound showed a 10-cm-diameter mass in the left adrenal gland with circumferential blood flow around the tumor. The choriocarcinoma recurrence was then diagnosed. Two courses of EMA/EP, which includes Etoposide (150 mg/m2), Actinomycin D (0.5 mg), Methotrexate (100 mg/m2) and Cisplatin (75 mg/m2) as chemotherapy regimen were given. The hard mass in the left adrenal gland measuring 10×9×7 cm had an obscure boundary (Figure 1D). Because fourth degree bone marrow suppression of preoperative EMA/EP occurred, we had to change chemotherapy regimen. The five courses of combined-drug chemotherapy, in which it contained Methotrexate [0.29 mg/(kg·d)], Fluorouracil [25 mg/(kg·d)], and Etoposide (100 mg/d) were performed (16) as adjuvant therapy. In the middle of the first cycle of treatment, adrenalectomy was performed with the help of urologists. The postoperative pathologic research revealed massive necrosis and hemorrhage with many poorly differentiated choriocarcinoma cells with immunohistochemical staining (Figure 1E,F,G,H). Her β-hCG returned to normal range (<1 mIU/L) eventually with negative CT scan result of the adrenal gland. The change trend of blood β-hCG level during illness was concluded in Figure 2. The patient was still under supervision in the outpatient department. The timeline figure was shown in Figure 3.
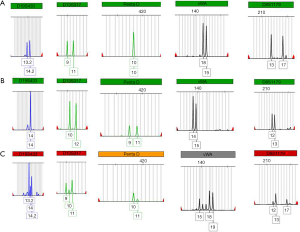
To distinguish primary and secondary choriocarcinoma of the left adrenal gland, a sample of the carcinoma and the couple’s blood was taken for further examination. All the examinations were carried out after the patient’s and her family’s agreement. The Microreader™ 21 (Direct) ID System (Suzhou Microread Genetics Co., Ltd., Suzhou, Jiangsu Province, China) was used for this analysis. For this case, a series of 6 formalin-fixed, paraffin-embedded tissue sections were prepared with 4-µm slide. DNA extraction was performed by following established protocols (17). Polymerase chain reaction amplification of 20 STR loci (CSF1PO, FGA, TH01, TPOX, vWA, D3S1358, D5S818, D7S820, D8S1179, D13S317, D16S539, D18S51, D21S11, Penta D, Penta E, D2S441, D2S1338, D12S391, D19S433 and D6S1043) and the amelogenin locus (for XY determination) was performed, with thermal cycling conditions and capillary electrophoresis carried out according to the manufacturer’s instructions. Electrophoresis was performed using an ABI 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Capillary electrophoresis data among the maternal and patrilineal blood samples and villous tissues were analyzed by Genemapper ID software (version 3.2.1; Applied Biosystems) to identify alleles at each locus.
The preserved tumor tissue and blood samples of the patient and her husband were sent for genetic comparison to determine the origin of the tumor. The genotype that was extracted from the paraffin-embedded tumor specimen was both maternal and patrilineal, showing a GC. The GC matched the patient’s and her husband’s blood sample for multiple probes (Figure 4). The complete analysis result is shown in Figure S1.
Ethical approval for this clinic study was obtained from the institutional review board of the First Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from the patient.
Discussion
Because the treatment and prognosis of primary and secondary choriocarcinoma differ, to distinguish these two types of carcinoma is necessary (1-6). Based on the review of related literatures, a series of measures were concluded for accurate diagnoses of primary choriocarcinoma. These measures can be divided into three groups: general analysis, microscopic analysis, and molecular detection.
General analysis includes sex, ability to be pregnant (or history of sex experience), and menophania. Sex seems to be the initial method for diagnoses since choriocarcinoma can occur in both sexes (2,18-20). And most clinicians believe that the diagnosis of primary choriocarcinoma is limited only to patients who are sexually immature, unable to conceive, or have never had sexual intercourse (3,4,10,13). Therefore, the patient in this case could not be definite according to her general analysis.
The pathologic origin of choriocarcinoma has not been confirmed yet. The most prevalent hypothesis shows that primary choriocarcinoma in extragonadal site might have been originated from the dedifferentiation of the adenocarcinoma (19). This hypothesis is based on both choriocarcinoma cells and adenocarcinoma cells present in many extragonadal choriocarcinoma cases (13,20). And the clinical manifestations are similar to both kinds of carcinoma (20). There have been cases of pure primary choriocarcinoma in the ovary (3,5,8,10). Hence, a presumption was made that pure primary choriocarcinoma might have originated from direct mutation of normal somatic cells. In this patient’s sample, there was no existence of adenocarcinoma. However, we cannot ignore the possibility that the appearance of adenocarcinoma might be complete metaplasia to choriocarcinoma with no residual adenocarcinoma (19). Therefore, a more appropriate molecular pathology way to find the origin of choriocarcinoma is needed.
Several researches have demonstrated that STR genetic analysis may help classify the tumor origin accurately, including most case reports and few case series (21-26). The latest and also the second largest case series, which through STR to analyze genotype patterns from tumoral and maternal formalin-fixed, paraffin-embedded (PPFE) tissue blocks in fifteen choriocarcinomas, illustrated that STR could serve as an useful examination to distinguish gestational choriocarcinoma from non-gestational choriocarcinoma (27). There has been no doubt that the quality and quantity of DNA extracted from biological samples is a key element for successful genetic analysis (28). And compared with blood samples, the process of PPFE could damage, modify, and degrade the DNA, and as a result, interfere with downstream genetic analysis such as STR analysis (29). Therefore, our study was designed to present the STR analysis through comparing the tumoral FFPE tissues, maternal and patrilineal fresh blood samples to discriminate gestational and non-gestational origin.
In this study, the sample of carcinoma from the patient as well as the peripheral blood sample of the patient and her husband was collected. The STR analysis demonstrated that the genotype extracted from the paraffin-embedded tumor specimen was both maternal and patrilineal. Hence, it can be concluded that the patient’s choriocarcinoma was related to pregnancy. Although the result did not meet our expectations, we describe a case in which a rare secondary adrenal choriocarcinoma was confirmed by STR analysis. There are literatures that describe DNA polymorphism analysis is also useful (5).
However, the treatment had some limitations. Because the tumor was huge, it would have been better if the tumor was removed after the first session of chemotherapy.
Above all, DNA analysis seems to be an accurate way to differentiate between GC and NGC. General analysis, microscopic analysis, and molecular detection should be done step by step for accurate diagnoses. An STR gene analysis was performed to confirm secondary choriocarcinoma. The patient was finally diagnosed with GC in the adrenal gland with lung metastasis.

Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval for this clinic study was obtained from the institutional review board of the First Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang L, Sookram J, Wilson-Smith R. A rare case of choriocarcinoma after a positive pregnancy test in a postmenopausal woman. Int J Gynaecol Obstet 2018;143:388-9. [Crossref] [PubMed]
- Hayashi S, Abe Y, Tomita S, et al. Primary non-gestational pure choriocarcinoma arising in the ovary: A case report and literature review. Oncol Lett 2015;9:2109-11. [Crossref] [PubMed]
- Goswami D, Sharma K, Zutshi V, et al. Nongestational pure ovarian choriocarcinoma with contralateral teratoma. Gynecol Oncol 2001;80:262-6. [Crossref] [PubMed]
- Park SH, Park A, Kim JY, et al. A case of non-gestational choriocarcinoma arising in the ovary of a postmenopausal woman. J Gynecol Oncol 2009;20:192-4. [Crossref] [PubMed]
- Tsujioka H, Hamada H, Miyakawa T, et al. A pure nongestational choriocarcinoma of the ovary diagnosed with DNA polymorphism analysis. Gynecol Oncol 2003;89:540-2. [Crossref] [PubMed]
- Sano R, Moriya T, Suzuki S, et al. Primary non-gestational uterine choriocarcinoma mimicking leiomyoma. Pathol Int 2019;69:160-4. [Crossref] [PubMed]
- Trastour C, Rahili A, Chevallier A, et al. Isolated bilateral adrenal choriocarcinoma. Lancet Oncol 2005;6:905-7. [Crossref] [PubMed]
- Lv L, Yang K, Wu H, et al. Pure choriocarcinoma of the ovary: a case report. J Gynecol Oncol 2011;22:135-9. [Crossref] [PubMed]
- Ahamed NA, Sait K, Anfnan N, et al. Gestational Choriocarcinoma Presenting with Lacrimal Gland Metastasis: A First Reported Case. Case Rep Obstet Gynecol 2015;2015:879538. [Crossref] [PubMed]
- Choi YJ, Chun KY, Kim YW, et al. Pure nongestational choriocarcinoma of the ovary: a case report. World J Surg Oncol 2013;11:7. [Crossref] [PubMed]
- Fukagawa A, Fujita N, Ohira K, et al. Primary hepatic choriocarcinoma in an 83-year-old woman. Pathol Int 2017;67:425-30. [Crossref] [PubMed]
- El Hasbani G, Balaghi A, Tarabine K, et al. Uterine choriocarcinoma diagnosed 11 years after menopause: A case report. Case Reports in Women's Health 2018;20:e00076. [PubMed]
- Koyanagi T, Fujiwara H, Usui H, et al. Ovarian nongestational choriocarcinoma and associated adenocarcinoma with the same germ cell origin determined by a molecular genetic approach: A case report. Pathol Int 2016;66:529-34. [Crossref] [PubMed]
- Yamamoto E, Ino K, Yamamoto T, et al. A pure nongestational choriocarcinoma of the ovary diagnosed with short tandem repeat analysis: case report and review of the literature. Int J Gynecol Cancer 2007;17:254-8. [Crossref] [PubMed]
- Zhou HY, Nguyen JK, Ganesan S, et al. Choriocarcinoma of the adrenal gland: A case report. Int J Surg Case Rep 2015;6C:92-4. [Crossref] [PubMed]
- Wang S, An R, Han X, et al. Combination chemotherapy with 5-fluorouracil, methotrexate and etoposide for patients with high-risk gestational trophoblastic tumors: a report based on our 11-year clinical experiences. Gynecol Oncol 2006;103:1105-8. [Crossref] [PubMed]
- Popiolek DA, Prinz MK, West AB, et al. Multiplex DNA short tandem repeat analysis. A useful method for determining the provenance of minute fragments of formalin-fixed, paraffin-embedded tissue. Am J Clin Pathol 2003;120:746-51. [Crossref] [PubMed]
- Hattori M, Imura H, Matsukura S, et al. Multiple-hormone producing lung carcinoma. Cancer 1979;43:2429-37. [Crossref] [PubMed]
- Chan GS, Ng WK, Chua DT, et al. Raised serum hCG in a male patient caused by primary jejunal choriocarcinoma. J Clin Pathol 1998;51:413-5. [Crossref] [PubMed]
- Kobayashi A, Hasebe T, Endo Y, et al. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer 2005;8:178-85. [Crossref] [PubMed]
- Aranake-Chrisinger J, Huettner PC, Hagemann AR, et al. Use of short tandem repeat analysis in unusual presentations of trophoblastic tumors and their mimics. Hum Pathol 2016;52:92-100. [Crossref] [PubMed]
- Zhao J, Xiang Y, Wan XR, et al. Molecular genetic analyses of choriocarcinoma. Placenta 2009;30:816-20. [Crossref] [PubMed]
- Fisher RA, Savage PM, MacDermott C, et al. The impact of molecular genetic diagnosis on the management of women with hCG-producing malignancies. Gynecol Oncol 2007;107:413-9. [Crossref] [PubMed]
- O'Neill CJ, Houghton F, Clarke J, et al. Uterine gestational choriocarcinoma developing after a long latent period in a postmenopausal woman: the value of DNA polymorphism studies. Int J Surg Pathol 2008;16:226-9. [Crossref] [PubMed]
- Wang Y, Yang Y, Teng F, et al. Pure nongestational uterine choriocarcinoma in a postmenopausal Chinese woman confirmed with short tandem repeat analysis. Am J Obstet Gynecol 2014;211:e1-3. [Crossref] [PubMed]
- Mello JB, Ramos Cirilo PD, Michelin OC, et al. Genomic profile in gestational and non-gestational choriocarcinomas. Placenta 2017;50:8-15. [Crossref] [PubMed]
- Zhang X, Yan K, Chen J, et al. Using short tandem repeat analysis for choriocarcinoma diagnosis: a case series. Diagn Pathol 2019;14:93. [Crossref] [PubMed]
- Wheeler A, Czado N, Gangitano D, et al. Comparison of DNA yield and STR success rates from different tissues in embalmed bodies. Int J Legal Med 2017;131:61-6. [Crossref] [PubMed]
- Okello JB, Zurek J, Devault AM, et al. Comparison of methods in the recovery of nucleic acids from archival formalin-fixed paraffin-embedded autopsy tissues. Anal Biochem 2010;400:110-7. [Crossref] [PubMed]