
Primary tumor resection and lymph node dissection improve survival in de novo metastatic pancreatic ductal adenocarcinoma: an inverse probability of treatment weighting analysis
Introduction
Pancreatic ductal carcinoma (PDAC) represents the fourth leading cause of cancer-related mortality worldwide (1). Surgery remains the only treatment option with the potential of cure for PDAC, and only a subset (20–30%) of patients have diseases at a locally resectable stage (2). Once distant metastases have occurred, only palliative chemotherapy or chemoradiation therapy is recommended by the guidelines (3). Despite progress in the treatment of metastatic PDAC (mPDAC), the prognosis remains dismal, with a median survival of only 8–11 months, even for patients receiving intensive chemotherapy with nab-paclitaxel/gemcitabine or FOLFIRINOX (4).
With the improved morbidity profile of pancreatic resection and metastasectomy and the potential for surgery to enhance survival, symptom control, and quality of life, primary tumor resection (PTR) with or without metastasectomy is gradually being attempted in select patients with mPDAC at some medical centers (5-7). For several other malignancies, such as gastrointestinal cancer and sarcoma, surgery and/or locoregional treatment within a multidisciplinary approach was considered to benefit select cases in terms of long-term disease control and survival (8,9). However, the beneficial role of PTR versus palliative chemotherapy for mPDAC in terms of long-term survival remains controversial. Moreover, the role of lymph node dissection (LND), which also contributes to the oncologic outcomes associated with the curative resection of non-metastatic PDAC, remains unclear (10).
Given that previous studies concerning this topic were primarily case series (5,6,11-15), this study aimed to analyze the impact of PTR and LND on OS and CSS in de novo mPDAC using a population-based database. Inverse probability of treatment weighting (IPTW) was applied to minimize the selection bias between the groups.
Methods
Database and population
This cohort study retrospectively analyzed data from the Surveillance Epidemiology and End Results (SEER) database (2010–2015), which sampled 26% of new cancers in the US. Ethics approval and informed consent were waived by the Chinese National Cancer Center Institutional Review Board, as no patient, physician, or hospital identifiers were examined.
Patients with de novo mPDAC diagnosed between 2010 and 2015 and treated with palliative chemotherapy or PTR plus chemotherapy were identified from the SEER database. Patients with PDAC were identified based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) morphological codes 8140, 8150, 8210, 8211, 8251, 8260, 8261, 8263, 8480, 8481, 8490, 8500, 8503, and topographical codes C25.0–C25.9. Only patients diagnosed between 2010 and 2015 were eligible because details regarding the specific locations of metastases (liver, lung, bone, and brain) have only been provided in the SEER database since 2010. The following patients were excluded: patients with any previous cancer diagnosis; patients with incomplete follow-up data; patients with an unknown cause of death; and patients with incomplete information regarding their PTR and LND status.
Study variables
Patient-related information included age at diagnosis, race, sex, and marital status. Tumor data included primary tumor location, clinical T stage, clinical nodal status, the number of positive lymph nodes, and the specific location of metastases. The T stage was restaged according to the American Joint Commission on Cancer 8th edition for pancreatic cancer (16). Specifically, T1 was defined as a tumor ≤2 cm in size; T2 was defined as a tumor 2–4 cm in size; T3 was defined as a tumor >4 cm in size; and T4 was defined as a tumor invading the celiac axis, the superior mesenteric artery, and/or common hepatic artery, regardless of size. Treatment-related factors included the number of examined lymph nodes, receipt of PTR, receipt of metastasectomy, and receipt of LND. LND was defined according to the combination of two variables: the scope of regional lymph node surgery and the number of lymph nodes retrieved. The primary endpoint in this study was overall survival (OS), and the secondary endpoint was cancer-specific survival (CSS).
Statistical analysis
We compared the baseline characteristics between patients who underwent PTR (PTR group) and those who received palliative chemotherapy (non-PTR group) among the entire cohort and between patients who underwent LND (LND group) and those who did not undergo LND (non-LND group) among the patients treated with PTR. Binary logistic regression analysis was used to identify independent predictive factors for PTR or LND. To minimize the treatment selection bias, significant differences in patient characteristics were adjusted using the IPTW method. Specifically, we first calculated the propensity score, i.e., the odds of receiving PTR, using a logistic regression model that included variables affecting treatment allocation and survival (age at diagnosis, sex, marital status, race, primary tumor location, clinical T stage, clinical nodal status, and location of metastases). The propensity score of receiving LND was calculated in a similar way.
We used the IPTW-adjusted Kaplan-Meier curves and log-rank test to compare OS and CSS between groups. IPTW-adjusted univariate and multivariate Cox regression models were used to estimate the effects of PTR and LND on survival. Covariates with univariate P<0.1 were selected for the logistic and Cox regression multivariable analyses. In this study, R version 3.5.1 (R Core Team 2018, Vienna, Austria) and SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) were used to perform all statistical analyses. Statistical significance was set at 2-sided P<0.05.
Results
Baseline characteristics
Overall, 10,036 patients with de novo mPDAC diagnosed between 2010 and 2015 met the inclusion criteria; 275 (2.7%) of these patients underwent PTR. The baseline features of the eligible patients before and after IPTW adjustment, stratified by receipt of PTR, are listed in Table 1. In a multivariable logistic regression analysis based on unadjusted patients, age at diagnosis, marital status, primary tumor location, clinical T category, clinical nodal status, bone metastasis, lung metastasis, and liver metastasis were independently associated with the odds of receiving PTR (Table 2). After IPTW adjustment, except for bone metastasis, the covariates did not differ significantly between the PTR group and the non-PTR group.
Table 1
| Variables | Before IPTW, n (%) | After IPTW (%) | |||||
|---|---|---|---|---|---|---|---|
| PTR (n=9,761) (97.3%) | Non-PTR (n=275) (2.7%) | P value | PTR | Non-PTR | P value | ||
| Age, years | |||||||
| >65 | 4,917 (50.4) | 115 (41.8) | 0.006 | 50.1 | 52.0 | 0.726 | |
| ≤65 | 4,844 (49.6) | 160 (58.2) | 49.9 | 48.0 | |||
| Gender | |||||||
| Female | 4,398 (45.1) | 120 (43.6) | 0.685 | 45.0 | 49.0 | 0.454 | |
| Male | 5,363 (54.9) | 155 (56.4) | 55.0 | 51.0 | |||
| Race | |||||||
| Black | 1,210 (12.4) | 30 (10.9) | 0.691 | 12.4 | 16.2 | 0.613 | |
| White | 7,816 (80.1) | 222 (80.7) | 80.1 | 75.4 | |||
| Other | 735 (7.5) | 23 (11.6) | 7.5 | 8.4 | |||
| Marital status | |||||||
| Never married | 1,370 (14.0) | 31 (11.3) | 0.021 | 14.0 | 17.6 | 0.248 | |
| Married | 6,063 (62.1) | 196 (71.3) | 62.4 | 63.7 | |||
| Previously married | 1,925 (19.7) | 39 (14.2) | 19.6 | 12.5 | |||
| Unknown | 403 (4.1) | 9 (3.3) | 4.1 | 6.1 | |||
| Primary tumor location | |||||||
| Head | 3,446 (35.3) | 147 (53.5) | <0.001 | 35.8 | 37.4 | 0.935 | |
| Body and tail | 3,790 (38.8) | 94 (34.2) | 38.7 | 37.0 | |||
| Other | 2,525 (25.9) | 34 (12.4) | 25.5 | 25.6 | |||
| Clinical nodal status | |||||||
| Negative | 4,880 (50.0) | 94 (34.2) | <0.001 | 49.6 | 57.2 | 0.388 | |
| Positive | 3,424 (35.1) | 174 (63.3) | 35.8 | 28.7 | |||
| Unknown | 1,457 (14.9) | 7 (2.5) | 14.6 | 14.1 | |||
| Clinical T category | |||||||
| T1 | 311 (3.2) | 3 (1.1) | <0.001 | 3.1 | 5.2 | 0.794 | |
| T2 | 2,626 (26.9) | 128 (46.5) | 27.4 | 25.2 | |||
| T3 | 2,375 (24.3) | 88 (32.0) | 24.5 | 26.9 | |||
| T4 | 2,085 (21.4) | 37 (13.5) | 21.1 | 18.4 | |||
| Tx | 2,364 (24.2) | 19 (6.9) | 23.7 | 24.4 | |||
| Bone metastasis | |||||||
| No | 8,756 (89.7) | 263 (95.6) | 0.005 | 89.9 | 92.3 | 0.018 | |
| Yes | 699 (7.2) | 7 (2.5) | 7.0 | 1.3 | |||
| Unknown | 306 (3.1) | 5 (1.8) | 3.1 | 6.4 | |||
| Lung metastasis | |||||||
| No | 7,404 (75.9) | 238 (86.5) | <0.001 | 76.2 | 79.3 | 0.366 | |
| Yes | 1,999 (20.5) | 34 (12.4) | 20.3 | 14.5 | |||
| Unknown | 358 (3.7) | 3 (1.1) | 3.6 | 6.1 | |||
| Brain metastasis | |||||||
| No | 9,388 (96.2) | 270 (98.2) | 0.213 | 96.2 | 93.6 | 0.547 | |
| Yes | 46 (0.5) | 1 (0.4) | 0.5 | 1.3 | |||
| Unknown | 327 (3.4) | 4 (1.5) | 3.3 | 5.0 | |||
| Liver metastasis | |||||||
| No | 2,189 (22.4) | 124 (45.1) | <0.001 | 23.1 | 20.9 | 0.749 | |
| Yes | 7,415 (76.0) | 149 (54.2) | 75.4 | 76.8 | |||
| Unknown | 157 (1.6) | 2 (0.7) | 1.6 | 2.3 | |||
IPTW, inverse probability of treatment weighting; PTR, primary tumor resection.
Table 2
| Variables | PTR* | LND§ | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (>65) (years) | 0.737 (0.572–0.949) | 0.018 | |||
| Marital status | |||||
| Never married | Reference | Reference | |||
| Married | 1.536 (1.036–2.279) | 0.033 | 0.980 (0.277–3.461) | 0.975 | |
| Previously married | 1.023 (0.626–1.672) | 0.929 | 0.245 (0.057–1.050) | 0.058 | |
| Unknown | 1.084 (0.502–2.336) | 0.838 | 0.125 (0.016–0.960) | 0.046 | |
| Primary tumor location | |||||
| Head | Reference | ||||
| Body and tail | 0.638 (0.485–0.838) | 0.001 | |||
| Other | 0.458 (0.309–0.678) | <0.001 | |||
| Clinical T category | |||||
| T1 | Reference | ||||
| T2 | 4.035 (1.262–12.903) | 0.019 | |||
| T3 | 3.336 (1.038–10.728) | 0.043 | |||
| T4 | 1.361 (0.412–4.489) | 0.613 | |||
| Tx | 1.066 (0.310–3.663) | 0.919 | |||
| Clinical nodal status | |||||
| Negative | Reference | Reference | |||
| Positive | 2.647 (2.037–3.440) | <0.001 | 16.706 (7.267–38.407) | <0.001 | |
| Unknown | 0.401 (0.184–0.877) | 0.022 | |||
| Bone metastasis | |||||
| No | Reference | ||||
| Yes | 0.340 (0.158–0.732) | 0.006 | |||
| Unknown | 1.900 (0.621–5.811) | 0.261 | |||
| Liver metastasis | |||||
| No | Reference | ||||
| Yes | 0.286 (0.221–0.369) | <0.001 | |||
| Unknown | 0.353 (0.069–1.801) | 0.211 | |||
| Lung metastasis | |||||
| No | Reference | ||||
| Yes | 0.390 (0.267–0.571) | <0.001 | |||
| Unknown | 0.307 (0.073–1.282) | 0.105 | |||
*, adjusted for age at diagnosis, marital status, primary tumor location, clinical T category, clinical nodal status, bone metastasis, liver metastasis, and lung metastasis; §, adjusted for age at diagnosis, race, marital status, clinical T category, and clinical nodal status. LND, lymph node dissection; OR, odds ratio; CI, confidence interval; PTR, primary tumor resection.
Among the 275 patients who underwent PTR, 217 (78.9%) also underwent LND. The baseline features of the patients treated with PTR before and after IPTW adjustment, stratified by receipt of LND, are presented in Table 3. The unweighted patient-based multivariable logistic regression analysis suggested that marital status, clinical T category, and clinical nodal status were independently associated with the odds of receiving LND (Table 2). After IPTW adjustment, except for bone metastasis, no residual significant imbalance was observed between the LND group and the non-LND group. Among patients who underwent LND, the median number of examined lymph nodes was 16 [interquartile range (IQR), 9–23]. Metastatic nodal disease was documented in a total of 245 patients (75.4%).
Table 3
| Variables | Before IPTW, n (%) | After IPTW (%) | |||||
|---|---|---|---|---|---|---|---|
| LND (n=217) (78.9%) | Non-LND (n=58) (21.1%) | P value | LND | Non-LND | P value | ||
| Age, years | |||||||
| >65 | 84 (38.7) | 31 (53.4) | 0.061 | 37.4 | 55.5 | 0.199 | |
| ≤65 | 133 (61.3) | 27 (46.6) | 62.6 | 44.5 | |||
| Gender | |||||||
| Female | 89 (41.0) | 31 (53.4) | 0.122 | 42.4 | 42.9 | 0.972 | |
| Male | 128 (59.0) | 27 (46.6) | 57.6 | 57.1 | |||
| Race | |||||||
| Black | 19 (8.8) | 11 (19.0) | 0.082 | 9.1 | 10.3 | 0.465 | |
| White | 180 (82.9) | 42 (72.4) | 82.0 | 71.4 | |||
| Other | 18 (8.3) | 5 (8.6) | 8.9 | 18.3 | |||
| Marital status | |||||||
| Never married | 25 (11.5) | 6 (10.3) | 0.009 | 11.1 | 10.1 | 0.971 | |
| Married | 162 (74.7) | 34 (58.6) | 72.2 | 74.5 | |||
| Previously married | 26 (12.0) | 13 (22.4) | 14.3 | 12.5 | |||
| Unknown | 4 (1.8) | 5 (8.6) | 2.4 | 3.0 | |||
| Primary tumor location | |||||||
| Head | 113 (52.1) | 34 (58.6) | 0.491 | 51.0 | 46.6 | 0.780 | |
| Body and tail | 78 (35.9) | 16 (27.6) | 36.8 | 33.6 | |||
| Other | 26 (12.0) | 8 (13.8) | 12.1 | 19.8 | |||
| Clinical nodal status | |||||||
| Negative | 52 (24.0) | 42 (72.4) | <0.001 | 34.4 | 32.1 | 0.430 | |
| Positive | 165 (76.0) | 9 (15.5) | 65.6 | 65.5 | |||
| Unknown | 0 (0) | 7 (12.1) | 0.0 | 2.4 | |||
| Clinical T category | |||||||
| T1 | 2 (0.9) | 1 (1.7) | 0.005 | 1.1 | 0.7 | 0.624 | |
| T2 | 104 (47.9) | 24 (41.4) | 47.0 | 32.7 | |||
| T3 | 75 (34.6) | 13 (22.4) | 32.2 | 42.6 | |||
| T4 | 27 (12.4) | 10 (17.2) | 13.2 | 18.2 | |||
| Tx | 9 (4.1) | 10 (17.2) | 6.5 | 5.8 | |||
| Bone metastasis | |||||||
| No | 207 (95.4) | 56 (96.6) | 0.229 | 96.1 | 99.3 | 0.029 | |
| Yes | 7 (3.2) | 0 (0.0) | 2.7 | 0.0 | |||
| Unknown | 3 (1.4) | 2 (3.4) | 1.2 | 0.7 | |||
| Lung metastasis | |||||||
| No | 190 (87.6) | 48 (82.8) | 0.136 | 88.5 | 90.7 | 0.728 | |
| Yes | 26 (12.0) | 8 (13.8) | 11.1 | 8.6 | |||
| Unknown | 1 (0.5) | 2 (3.4) | 0.4 | 0.7 | |||
| Brain metastasis | |||||||
| No | 214 (98.6) | 56 (96.6) | 0.317 | 98.8 | 99.3 | 0.586 | |
| Yes | 1 (0.5) | 0 (0.0) | 11.1 | 0.0 | |||
| Unknown | 2 (0.9) | 2 (3.4) | 0.4 | 0.7 | |||
| Liver metastasis | |||||||
| No | 104 (47.9) | 20 (34.5) | 0.129 | 46.3 | 45.0 | 0.956 | |
| Yes | 112 (51.6) | 37 (63.8) | 53.3 | 54.6 | |||
| Unknown | 1 (0.5) | 1 (1.7) | 0.4 | 0.4 | |||
| Metastasectomy | 39 (18.0) | 11 (19.0) | 1.000 | 18.7 | 9.2 | 0.089 | |
IPTW, inverse probability of treatment weighting; LND, lymph node dissection.
Survival outcomes
Overall, 9,447 patients (94.1%) died during the study period, and 9,213 (91.8% of all deaths) died of mPDAC. The median follow-up time was 41.0 [95% confidence interval (CI), 38.2–43.7] months.
PTR and survival in mPDAC
In the IPTW-adjusted Kaplan-Meier analysis, PTR was associated with a significant OS benefit [hazard ratio (HR), 0.456; 95% CI, 0.442–0.470; P<0.001] and CSS benefit (HR, 0.457; 95% CI, 0.444–0.472; P<0.001). The IPTW-adjusted median OS was 13 (IQR, 13–14) versus 6 (IQR, 6–7) months, and the median CSS was 13 (IQR, 13–14) versus 7 (IQR, 6–7) months for the PTR group and non-PTR group, respectively (Figure 1A,B). In the IPTW-adjusted multivariate Cox proportional hazards regression analysis, PTR was independently associated with an OS benefit (HR, 0.504; 95% CI, 0.441–0.576; P<0.001) and a CSS benefit (HR, 0.505; 95% CI, 0.441–0.579; P<0.001) (Table 4).
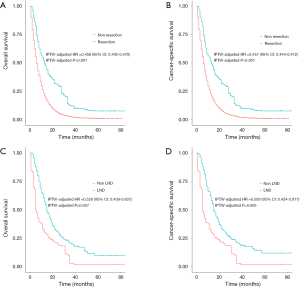
Table 4
| Variables | OS* | CSS* | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (>65) (years) | 1.436 (1.393–1.480) | <0.001 | 1.460 (1.415–1.506) | <0.001 | |
| Gender (female) | 0.894 (0.867–0.922) | <0.001 | 0.898 (0.870–0.926) | <0.001 | |
| Race | |||||
| Black | Reference | Reference | |||
| White | 1.061 (1.014–1.111) | 0.011 | 1.067 (1.018–1.117) | 0.005 | |
| Other | 1.304 (1.217–1.396) | <0.001 | 1.331 (1.242–1.427) | <0.001 | |
| Marital status | |||||
| Never married | Reference | Reference | |||
| Married | 0.688 (0.659–0.718) | <0.001 | 0.683 (0.654–0.713) | <0.001 | |
| Previously married | 0.907 (0.860–0.956) | <0.001 | 0.869 (0.824–0.918) | <0.001 | |
| Unknown | 0.862 (0.793–0.938) | 0.001 | 0.857 (0.788–0.933) | <0.001 | |
| Primary tumor location | |||||
| Head | Reference | Reference | |||
| Body and tail | 0.882 (0.850–0.915) | <0.001 | 0.871 (0.840–0.904) | <0.001 | |
| Other | 0.927 (0.890–0.966) | <0.001 | 0.933 (0.896–0.973) | 0.001 | |
| Clinical T category | |||||
| T1 | Reference | Reference | |||
| T2 | 0.844 (0.772–0.923) | <0.001 | 0.832 (0.761–0.910) | <0.001 | |
| T3 | 0.811 (0.741–0.887) | <0.001 | 0.793 (0.725–0.868) | <0.001 | |
| T4 | 0.716 (0.654–0.784) | <0.001 | 0.694 (0.634–0.761) | <0.001 | |
| Tx | 1.138 (1.040–1.244) | <0.001 | 1.122 (1.026–1.227) | <0.001 | |
| Clinical nodal status | |||||
| Negative | Reference | Reference | |||
| Positive | 1.132 (1.094–1.172) | <0.001 | 1.121 (1.083–1.161) | <0.001 | |
| Unknown | 1.295 (1.236–1.356) | <0.001 | 1.296 (1.237–1.358) | <0.001 | |
| Bone metastasis | |||||
| No | Reference | Reference | |||
| Yes | 1.110 (1.030–1.196) | 0.006 | 1.114 (1.033-1.201) | 0.005 | |
| Unknown | 1.053 (0.871–1.274) | 0.591 | 1.062 (0.876-1.288) | 0.541 | |
| Liver metastasis | |||||
| No | Reference | Reference | |||
| Yes | 1.212 (1.167–1.259) | <0.001 | 1.205 (1.159–1.252) | <0.001 | |
| Unknown | 0.852 (0.735–0.989) | 0.035 | 0.848 (0.730-0.985) | 0.030 | |
| Brain metastasis | |||||
| No | Reference | Reference | |||
| Yes | 1.110 (1.030–1.196) | 0.037 | 1.233 (0.989–1.538) | 0.063 | |
| Unknown | 1.054 (0.871–1.274) | 0.002 | 1.298 (1.091–1.543) | 0.003 | |
| Lung metastasis | |||||
| No | Reference | Reference | |||
| Yes | 1.108 (1.064–1.153) | <0.001 | 1.113 (1.069–1.160) | <0.001 | |
| Unknown | 0.984 (0.866–1.119) | 0.803 | 0.995 (0.874–1.132) | 0.926 | |
| PTR | 0.483 (0.468–0.498) | <0.001 | 0.485 (0.470–0.500) | <0.001 | |
*, all variables collected in this study was with a univariate P value <0.1. IPTW, inverse probability of treatment weighting; OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval, PTR, primary tumor resection.
LND and survival in mPDAC
In the IPTW-adjusted Kaplan-Meier analysis, LND was associated with a significant OS benefit (HR, 0.526; 95% CI, 0.439–0.631; P=0.007) and CSS benefit (HR, 0.509; 95% CI, 0.424–0.611; P=0.005). The IPTW-adjusted median OS was 15 (IQR, 14–17) versus 5 (IQR, 5–7) months, and the median CSS was 15 (IQR, 14–18) versus 5 (IQR, 5–7) months for the LND group and non-LND group, respectively (Figure 1C,D). In IPTW-adjusted multivariate Cox proportional hazards regression analysis, LND was independently associated with an OS benefit (HR, 0.286; 95% CI, 0.228–0.358; P<0.001) and a CSS benefit (HR, 0.264; 95% CI, 0.209–0.333; P<0.001) (Table 5).
Table 5
| Variables | OS* | CSS* | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| LND | 0.286 (0.228–0.358) | <0.001 | 0.264 (0.209–0.333) | <0.001 | |
| Age (>65) (years) | 1.540 (1.269–1.868) | <0.001 | 1.583 (1.301–1.926) | <0.001 | |
| Race | |||||
| Black | Reference | Reference | |||
| White | 1.112 (0.785–1.575) | 0.552 | 1.112 (0.780–1.586) | 0.558 | |
| Other | 2.065 (1.353–3.151) | 0.001 | 2.079 (1.354–3.192) | 0.001 | |
| Clinical nodal status | |||||
| Negative | Reference | Reference | |||
| Positive | 2.034 (1.599–2.589) | <0.001 | 2.122 (1.656–2.718) | <0.001 | |
| Unknown | 0.947 (0.425–2.111) | 0.895 | 0.941 (0.421–2.102) | 0.882 | |
| Marital status | |||||
| Never married | Reference | Reference | |||
| Married | 1.649 (1.197–2.273) | 0.002 | 1.631 (1.181–2.252) | 0.003 | |
| Previously married | 0.999 (0.670–1.489) | 0.996 | 0.886 (0.589–1.332) | 0.560 | |
| Unknown | 0.899 (0.430–1.878) | 0.776 | 0.851 (0.406–1.782) | 0.668 | |
| Clinical T category | |||||
| T1 | Reference | Reference | |||
| T2 | 0.624 (0.165–2.358) | 0.487 | 0.600 (0.158–2.277) | 0.453 | |
| T3 | 0.793 (0.209–3.007) | 0.733 | 0.754 (0.198–2.869) | 0.679 | |
| T4 | 0.173 (0.044–0.690) | 0.013 | 0.153 (0.038–0.614) | 0.008 | |
| Tx | 0.740 (0.185–2.955) | 0.669 | 0.717 (0.179–2.873) | 0.638 | |
| Bone metastasis | |||||
| No | Reference | Reference | |||
| Yes | 0.434 (0.162–1.167) | 0.098 | 0.431 (0.160–1.160) | 0.096 | |
| Unknown | 1.460 (0.590–3.610) | 0.413 | 1.462 (0.590–3.623) | 0.411 | |
| Metastasectomy | 0.700 (0.516–0.950) | 0.022 | 0.685 (0.502–0.935) | <0.001 | |
*, all variables collected in this study was with a univariate P value <0.1. IPTW, inverse probability of treatment weighting; LND, lymph node dissection; OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval.
Lymph node status and survival in mPDAC
The multivariate analysis results suggested that nodal status was an independent prognostic factor of OS and CSS (Table 4). However, the number of examined lymph nodes was not associated with OS (HR 1.000; 95% CI, 0.991–1.009, P=0.960) or CSS (HR 0.953; 95% CI, 0.991–1.009, P=0.953). There was no significant difference between patients with <16 and ≥16 examined lymph nodes in terms of OS (P=0.728) or CSS (P=0.733).
Discussion
This SEER-based study represents one of the latest attempts to investigate the survival benefit of PTR and the first study to investigate LND among de novo mPDAC patients. Our results suggested that PTR and LND were independently associated with a significant survival benefit compared to palliative chemotherapy in patients with de novo mPDAC.
With the decreasing surgery-related morbidity over the past decades, mortality rates lower than 5%, and the emergence of potent chemotherapy regimens such as nab-paclitaxel and FOLFIRINOX, PTR with or without preoperative chemotherapy has been gradually adopted as a potential treatment option for mPDAC. Our findings suggested that PTR was associated with survival benefit compared to palliative chemotherapy. Similar to our results, Tao et al. identified 467 patients with mPDAC who had PTR from the SEER database. They concluded that local treatment has a primary benefit on both CSS and OS in patients with mPDAC (17). Bahra et al. suggested that the survival of 45 patients who had primary cytoreductive surgery followed by chemotherapy was superior to that of patients who received palliative chemotherapy if a tumor-free margin could be achieved (7). The potential mechanism underlying the beneficial role of PTR may be as follows. PDAC is a tumor with more than half of the tumor volume being stroma characterized by extensive fibrosis and extracellular matrix deposition (18,19). The dense stroma mediates the chemotherapy-resistant property of pancreatic cancers by blocking tumor cells from the effects of chemotherapeutic agents. Thus, stroma-mediated chemotherapy insensitivity is one of the important mechanisms underlying the poor prognosis of patients with PDAC (20,21). It was observed that unconformity existed in the response to chemotherapy between the primary lesion and the metastases. Dense stroma within the primary tumor versus the metastases may be the reason for this discrepancy in tumor response. Thus, PTR could theoretically improve the chemotherapy sensitivity of mPDAC by removing the primary tumor with dense stroma even when complete removal of all tumor lesions is not possible.
Relevant literature regarding the benefits of PTR for de novo mPDAC in terms of long-term survival is very limited, and suggestions on this issue remain controversial. Gleisner et al. retrospectively analyzed data from 22 patients who underwent upfront surgical resection for PDAC with synchronous liver disease (22). In their results, simultaneous resection of primary lesions and liver metastases did not result in a better prognosis, even for select patients with a low burden of liver metastasis. Tachezy et al. also identified 69 similar patients who underwent simultaneous resection of the pancreas and liver metastasis. The OS was improved in patients who underwent simultaneous resection (median 14 vs. 8 months, P<0.001) (14). Tumor resection was the only independent predictor of OS in the multivariate analysis. Based on these studies, surgery could be discreetly considered as a treatment strategy for super select patients with mPDAC.
To the best of our knowledge, this is the first study to investigate the beneficial role of LND in terms of long-term survival for patients with mPDAC. For patients treated with PTR, LND was significantly associated with better OS and CSS. The oncologic benefit of LND in non-metastatic PDAC could be justified by the complete removal of cancer. However, it was interesting to observe a beneficial prognostic role of LND in the resection of the primary tumor for mPDAC. LND usually did not bear prognostic benefits in patients with mPDAC whose metastatic lesions were largely left intact. A mechanism associated with cancer immunity may be responsible for the beneficial role of LND in mPDAC. It was reported that there was a balance between antitumor and protumor immunity inside the tumor-draining lymph nodes (TDLN). As the cancer advanced, the metastatic tumor cells inside the TDLN tipped the balance toward protumor immunity and converted the immunologic microenvironment of the TDLN to a tolerogenic milieu (23). Preclinical studies have suggested that the accumulation of Foxp3+ T regulatory cells in the TDLN inhibited the function of CD8+ T cells (24). Foxp3+ T cell-dependent dendritic cell death in the TDLN inhibited the onset of CD8+ T cell responses (25). Therefore, the removal of regional lymph nodes, even if they are negative, may also reverse protumor immunity, resulting in a better prognosis.
There are some limitations to our study. First, this study was retrospective and thus could not be free of selection bias. The selection criteria for surgery were unknown in the SEER database, although they must be related to the amount of tumor burden. Indeed, the SEER database lacks information regarding the number and distribution of metastatic lesions. Second, the SEER database did not contain information on comorbidities, performance status, and chemotherapeutic agents, which may confound the evaluation of the actual effects of surgery. Finally, the SEER database also lacks information about surgical margin status and complication rates, which are instrumental in the decision to perform a major operation.
Conclusions
Although this is a retrospective study, the results suggest that PTR and LND were independent prognostic factors that prolonged OS and CSS in de novo mPDAC patients. However, these findings must be validated in prospective randomized studies.
Acknowledgments
The authors appreciate the Surveillance, Epidemiology, and End Results database in providing high quality clinical data for our research.
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital has reviewed and approved this study, and has also agreed that individual patient consent was not required to report clinical outcomes alone (No. 17-156/1412).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; [Crossref] [PubMed]
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Prim 2016;2:16022. [Crossref] [PubMed]
- Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw 2019;17:603-5. [PubMed]
- Reni M, Zanon S, Peretti U, et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): a randomised phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:691-7. [Crossref] [PubMed]
- Hackert T, Niesen W, Hinz U, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol 2017;43:358-63. [Crossref] [PubMed]
- Frigerio I, Regi P, Giardino A, et al. Downstaging in Stage IV Pancreatic Cancer: A New Population Eligible for Surgery? Ann Surg Oncol 2017;24:2397-403. [Crossref] [PubMed]
- Bahra M, Pratschke J, Klein F, et al. Cytoreductive surgery for pancreatic cancer improves overall outcome of gemcitabine-based chemotherapy. Pancreas 2015;44:930-6. [Crossref] [PubMed]
- Wigge S, Heißner K, Steger V, et al. Impact of surgery in patients with metastatic soft tissue sarcoma: A monocentric retrospective analysis. J Surg Oncol 2018;118:167-76. [Crossref] [PubMed]
- Chawla A, Williams RT, Sich N, et al. Pancreaticoduodenectomy and metastasectomy for metastatic pancreatic neuroendocrine tumors. J Surg Oncol 2018;118:983-90. [Crossref] [PubMed]
- Eskander MF, de Geus SWL, Kasumova GG, et al. Evolution and impact of lymph node dissection during pancreaticoduodenectomy for pancreatic cancer. Surgery 2017;161:968-76. [Crossref] [PubMed]
- Wright GP, Poruk KE, Zenati MS, et al. Primary Tumor Resection Following Favorable Response to Systemic Chemotherapy in Stage IV Pancreatic Adenocarcinoma with Synchronous Metastases: a Bi-institutional Analysis. J Gastrointest Surg 2016;20:1830-5. [Crossref] [PubMed]
- Furuse J, Shibahara J, Sugiyama M. Development of chemotherapy and significance of conversion surgery after chemotherapy in unresectable pancreatic cancer. J Hepatobiliary Pancreat Sci. 2018;25:261-8. [Crossref] [PubMed]
- Ghidini M, Petrillo A, Salati M, et al. Surgery or Locoregional Approaches for Hepatic Oligometastatic Pancreatic Cancer: Myth, Hope, or Reality? Cancers (Basel) 2019;11:1095. [Crossref] [PubMed]
- Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016;160:136-44. [Crossref] [PubMed]
- Antoniou E, Margonis GA, Sasaki K, et al. Is resection of pancreatic adenocarcinoma with synchronous hepatic metastasis justified? A review of current literature. ANZ J Surg 2016;86:973-7. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CFD, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017;265:185-91.
- Tao L, Yuan C, Ma Z, et al. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: a population-based study. Cancer Manag Res 2017;9:471-9. [Crossref] [PubMed]
- Wang WQ, Liu L, Xu HX, et al. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br J Surg 2016;103:1189-99. [Crossref] [PubMed]
- Dougan SK. The Pancreatic Cancer Microenvironment. Cancer J 2017;23:321-5. [Crossref] [PubMed]
- Dauer P, Nomura A, Saluja A, et al. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology 2017;17:7-12. [Crossref] [PubMed]
- Rajabpour A, Rajaei F, Teimoori-Toolabi L. Molecular alterations contributing to pancreatic cancer chemoresistance. Pancreatology. 2017;17:310-20. [Crossref] [PubMed]
- Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 2007;110:2484-92. [Crossref] [PubMed]
- Balsat C, Blacher S, Herfs M, et al. A specific immune and lymphatic profile characterizes the pre-metastatic state of the sentinel lymph node in patients with early cervical cancer. Oncoimmunology 2017;6:e1265718. [Crossref] [PubMed]
- Deng L, Zhang H, Luan Y, et al. Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin Cancer Res. 2010;16:4105-12. [Crossref] [PubMed]
- Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity 2010;32:266-78. [Crossref] [PubMed]


