PD-L1 for gallbladder cancer: case report
Introduction
The biliary tract cancer (BTC) is very rare in China, they contain gallbladder carcinoma (GBC) and cholangiocarcinoma (CCA). Gallbladder cancer (GBC) is the most common malignant tumor in BTC, and the incidence rate is fifth in digestive tract tumors (1). About 90% of patients with BTC are detected at advanced stages. With local advance and metastasis, the median survival time is less than 1 year (2). From Dec. 2018 to Feb. 2020, no obvious signs of progress have been shown in 14 months. The patient is still being treated and the situation is stable recently. This shows that immunotherapy (pembrolizumab) combined with first-line chemotherapy (oxaliplatin + capecitabine) for GBC is of great clinic benefit. We present the following case in accordance with the CARE-Guideline (3).
Case presentation
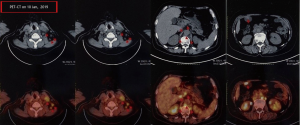
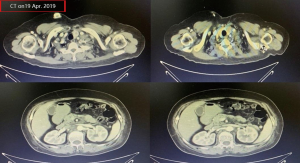
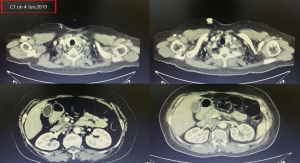
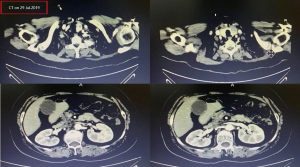
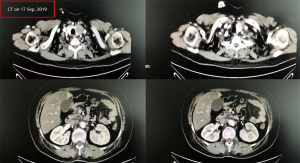
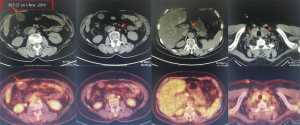
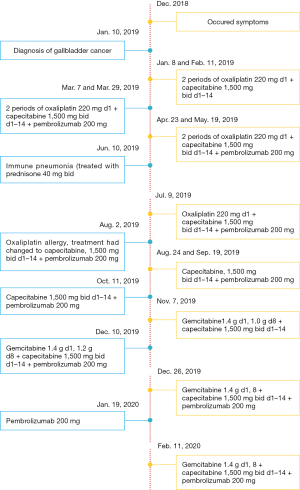
A female patient, 61 years old, without related family genetic history, was appeared dull pain in the upper abdomen, occasionally poor defaecating in Dec. 2018. The PET-CT on Jan. 10, 2019 showed: abnormally high FDG metabolism in the gallbladder body, the abnormally high of FDG metabolism in left neck (II−IV area), left clavicle area, bilateral medial diaphragmatic angle, hepatic portal area, retroperitoneal and multiple lymph nodes near the left iliac vessels, which was suspicious malignant lesions: GBC with lymph nodes metastasis? Besides, suspicious lymphoma with organ infiltration (Figure 1) was observed. Tumor markers showed CEA 42.32 ng/mL, CA724 45.62 U/mL and CA153 70.39 U/mL. Left cervical lymph node biopsy on Jan. 10 (left neck) invasive/metastatic adenocarcinoma. The primary clinical impression was primary malignant tumor of the gallbladder with lymph nodes metastasis, lymphoma with gallbladder infiltration to be excluded. Genetic testing: a total of 12 genetic variants, of which 3 have clear or potentially clinically significant variations, CCNE1, and ERBB2 gene copy number amplification, TP53 p. Asp228fs; tumor mutation burden (TMB) was 7.9 mutations/mb and microsatellite stabilization (MSS) was confirmed. Immunohistochemistry: PD-L1 expression ratio of tumor cells: 5% + (low expression). On Jan. 18 and Feb. 11, the patient received two periods of chemotherapy: oxaliplatin 220 mg d1 + capecitabine 1,500 mg bid d1–14. On Mar. 6, the review of CT: nodules in the bottom wall of the gallbladder; thickening of the local wall of the rectum; multiple swelling lymph nodes in the left clavicle, left neck, and retroperitoneum. Efficacy evaluation of disease: stable disease (SD). On Mar. 7 and Mar. 29, the patient received two periods of chemotherapy: oxaliplatin 220 mg d1 + capecitabine 1,500 mg bid d1–14, combined with pembrolizumab 200 mg. On Apr. 19, a CT scan showed that the gallbladder wall was unevenly thickened, and there are multiple swelling lymph nodes in the left neck, clavicular area and posterior peritoneum. Some lymph nodes were smaller than those on Mar. 6, the rectal wall is thickened, similar to the previous one (Figure 2). Efficacy evaluation of disease: partial response (PR). On Apr. 23 and May. 19, the patient received two periods of chemotherapy, oxaliplatin 220 mg d1 + capecitabine 1,500 mg bid d1–14, combined with pembrolizumab 200 mg. On Jun. 4, a CT showed: after the treatment of GBC, the gallbladder wall was unevenly thickened, and the left neck, clavicular area and posterior peritoneum multiple lymph nodes were smaller than before (Apr. 19, 2019) (Figure 3). Efficacy evaluation of disease: complete response (CR). On Jun. 10, an X-ray scan showed increased lung texture on both sides without chest tightness or cough, which considering immune pneumonia, treated with prednisone 40 mg bid, after it, the situation of disease got improved. On Jul. 9, the patient received one period of chemotherapy: oxaliplatin 220 mg d1 + capecitabine 1,500 mg bid d1–14, combined with the pembrolizumab 200 mg. On Jul. 29, a CT scan revealed the gallbladder wall is unevenly thickened, and the left neck, clavicle area and posterior peritoneum multiple lymph nodes are similar to the previous one (Jun. 4, 2019) (Figure 4). On Aug. 2, Aug. 24, Sep.19 and Oct.11, the patient received four periods of capecitabine 1,500 mg bid d1–14 plus pembrolizumab 200 mg (On Aug. 2, the patient appeared oxaliplatin allergy, so the treatment had changed to capecitabine single-agent chemotherapy). On Sep. 17, CT showed: uneven thickening of the gallbladder wall, multiple lymph nodes in the left neck, clavicle region and posterior peritoneum, which were roughly similar to the previous one (Jul. 29, 2019) (Figure 5). Comparing PET-CT on November 5 (Figure 6) with PET-CT on January 10 (Figure 1), the gallbladder lesions were significantly smaller and the metabolism was reduced, and the metastatic lymph nodes in the left neck, abdominal cavity and retroperitoneum were significantly smaller and the metabolism was reduced. Pulmonary nodules basically disappeared. On Nov. 7, the patient received gemcitabine 1.4 g d1, 1.0 g d8+capecitabine 1,500 mg bid d1–14. On Dec. 10, the patient received gemcitabine1.4 g d1, 1.2 g d8 + capecitabine 1,500 mg bid d1–14 plus pembrolizumab 200 mg. On Dec. 26, the patient received gemcitabine 1.4 g d1, 8 + capecitabine 1,500 mg bid d1–14 plus pembrolizumab 200 mg. On Jan. 19, 2020, the patient received pembrolizumab 200 mg. On Feb.11, 2020, the patient received gemcitabine1.4 g d1, 8 + capecitabine 1,500 mg bid d1–14 plus pembrolizumab 200 mg. And the patient’s tumor marker CEA levels are declining. The patient’s condition is stable recently.
Figure 7 summarizes the treatment process of the patients.
Discussion
The incidence of GBC has large difference in different regions. In Chile and western Argentina, age-standardized mortality rate (ASMR) is high, and in Western countries such as Peru and Ecuador, age-standardized incidence rate (ASIR, equivalent to ASMR) is high. The statistic indicated that people in a range of areas near the Andes are more likely to develop GBC (4). There are also researches indicated that the Indian Ganges plain in India, the Mapuche Indians in Chile and South America are the most affected areas (5). A study of BTC based on the Shanghai population shows that the mortality rate of GBC is increasing, and the family history of biliary stones in first-degree relatives increases the risk of biliary stones and the risk of BTC (6).
For the genomics of GBC, a relevant study showed that the occurrence of GBC may be related to APC, ARID1A, BRAF, CDKN2A/B, CTNNB1, HER family gene, RAS, PI3K/AKT/mTOR pathway gene, PBRM1, SMAD4, TP53 (7).
To the early stage of GBC, some studies have shown that early surgery is the only way to cure this cancer (8). For advanced GBC, the earlier ABC-O1 trial in UK showed gemcitabine (Gem) as group A, gemcitabine plus cisplatin (GemCis) as group B, and GemCis group for 6 months of progression-free survival (PFS) was higher (Group A and Group B were 45.5 vs. 57.1%, respectively) (9). A landmark Phase III trial of ABC-02 based on ABC-01 showed that the program of GemCis is effective in patients with advanced GBC who cannot undergo palliative care and is established as standard first-line treatment for advanced GBC [there is related analysis of Meta to strengthen the rationality of the program (10)]. There are some studies prove: in the 410 patients of BTC, after the median follow-up of 9.2 months and 398 patients died, the median overall survival (OS) of GemCis was 11.7 months, the median OS of gemcitabine (Gem) was 8.1 months, and GemCis was longer than the single drug Gem by 3.6 months (P<0.001). Patients treated with GemCis are more likely to have long-term survival (long-term survival is defined as patients who have survived for more than two years) (11). Some scholars selected 40 patients with BTC(including 6 cases of GBC, 4 cases of extrahepatic CCA and 30 cases of intrahepatic CCA), 2 of whom did not receive any treatment, and the remaining 38 patients had previously received three systemic treatments, and 15 patients (38%) underwent surgical resection, while 13 (33%) received radiation therapy, while 3 (8%) received chemoembolization, while 23% received targeted therapy, the most common targeted drug is angiogenesis inhibitors, emphasizing the role of targeted therapy in the treatment of advanced BTC(12). However, the total sample size is small, and most patients receive other treatments than targeted therapy. This research cannot reflect the absolute significance of targeted therapy for advanced BTC, it also does not reflect whether targeted therapy can improve OS.
This patient of advanced GBC has PD-L1-positive. We used standard first-line treatment combined with pembrolizumab to treat this patient and achieved good effect. This patient with immune pneumonia after 6 periods of systemic chemotherapy combined with immunotherapy may be related to the efficacy of this program. In fact, pembrolizumab has achieved good results in a variety of cancers, including high microsatellite instability (MSI-H) cancers (13). In the Phase Ib trial of KEYNOTE-028, it was shown that pembrolizumab was well tolerated and had strong antitumor activity against PD-L1-positive advanced BTC (14,15). The results of Phase II trial KEYNOTE-158 indicate that regardless of PD-L1 expression, pembrolizumab has a long-lasting anti-tumor effect on patients with advanced BTC, with manageable toxicity and high safety (16). Professor Shukui Qin from the All-Army Cancer Center of the General Hospital of the Eastern Theater of the People's Liberation Army made a combination of camrelizumab and FOFOX4 (fluorouracil + leucovorin + oxaliplatin) or GEMOX (gemcitabine + oxaliplatin) systemic chemotherapy as Phase II study of first-line treatment for hepatocellular carcinoma (HCC) or BTC: 81 patients with advanced HCC or BTC who did not receive systemic therapy from 27 Apr. 2017 to 31 Oct. 2018 (HCC: 34, BTC: 47) as observation objects, they are received camrelizumab (3 mg/kg, q2w), combined with FOLFOX4 or GEMOX, the primary end point was objective response rate (ORR, HCC vs. BTC =26.5% vs. 7.0%), secondary end points including disease control rate (DCR, HCC vs. BTC =79.4% vs. 67.4%), median progression-free survival time (mPFS, HCC vs. BTC =5.5 months vs. not yet achieved), safety (controllable), etc. Because of the limited treatment of patients with advanced HCC and BTC, considering the immunogenic effects of oxaliplatin, camrelizumab combined with oxaliplatin-based chemotherapy may be get more benefits of clinic. Professor Shu from Jiangsu Provincial People’s Hospital made camrelizumab (SHR-1210) in combination with GEMOX for the treatment of BTC: recruited 27 patients with BTC who were treated for more than 2 months. The age span is 45–75 years old (CCA vs. GBC =16 vs. 11), given camrelizumab (3mg/kg, total dose ≤200 mg, d1/2w) + gemcitabine (800 mg /m2, d1/2w) + oxaliplatin (85 mg/m2, d2/2w), from Feb. 2018 to Dec. 2018, the results show patients with GBC had a higher ORR (63.64% vs. 33.33%, P=0.23) and a higher TMB (TMB,8.1 mut/Mb vs. 5.4 mut/Mb, P=0.33). Patients with high TMB (>8.6 mut/Mb) had significantly higher ORR than patients with low TMB (100% vs. 26%, P=0.0294). The study indicated that the first-line immunotherapy combined with chemotherapy for advanced BTC reached SD + PR + CR are 46.15%, 46.15% and 7.70%, so immunotherapy treatment may be a possible approach for BTC. The related studies have shown that PD-L1 expression predict the response of various types of tumors to pembrolizumab, which may help to determine patients with higher response to immunotherapy (17). The relevant studies have stated that pembrolizumab is effective in the treatment of cancers such as non-small cell lung cancer and melanoma (18,19). Nowadays, camrelizumab has been put into clinical use, and related researches suggest that the immunotherapy agent’s toxicity and safety can be managed in advanced tumors (20). In this case, a standard first-line treatment combined with immunotherapy was used, which is relatively novel. However, the patient developed oxaliplatin allergy during the eighth chemotherapy. Therefore, he could only be treated with capecitabine combined pembrolizumab, and then treated with gemcitabine + capecitabine combined pembrolizumab. In terms of late treatment, standard first-line chemotherapy is not fully followed, but the results were fine, and there were no signs of progress.
The author thinks that the treatment of BTC is still based on surgery, and early surgery combined with other adjuvant treatment is the only way to cure this cancer. And GemCis is the standard first-line treatment for advanced BTC, and there is no recommended second-line treatment for this cancer. The experiments of KN-028 and KN-158 showed that immunotherapy can bring more benefits to patients with advanced BTC. In the BTC, the standard first-line treatment program combined with immunotherapy is worth looking forward to.
After treatment, the patient’s dull pain in the upper abdomen has improved. And the diet and sleep are normal. The patient expressed great expectations for this emerging therapy.
Acknowledgments
Funding: All authors report grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.25). DZ reports grants from National Natural Science Foundation of China (81770212), outside the submitted work; HY reports grants from Key Program of the Changzhou Commission of Health (ZD201709), outside the submitted work; XL reports grants from Program for Young Talents of the Changzhou Commission of Health (QN201902), outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baillie J. Tumors of the Gallbladder and Bile Ducts. J Clin Gastroenterol 1999;29:14-21. [Crossref] [PubMed]
- Shah UA, Nandikolla AG, Rajdev L. Immunotherapeutic Approaches to Biliary Cancer. Curr Treat Options Oncol 2017;18:44. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Arroyo GF, Gentile A, Parada LA. Gallbladder cancer: South American experience. Chin Clin Oncol 2016;5:67. [Crossref] [PubMed]
- Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 2017;23:3978-98. [Crossref] [PubMed]
- Hsing AW, Bai Y, Andreotti G, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer 2007;121:832-8. [Crossref] [PubMed]
- Sicklick JK, Fanta PT, Shimabukuro K, et al. Genomics of gallbladder cancer: the case for biomarker-driven clinical trial design. Cancer Metastasis Rev 2016;35:263-75. [Crossref] [PubMed]
- Di Carlo I, Toro A. Gallbladder cancer: results achieved and future challenges. Future Oncol 2017;13:209-11. [Crossref] [PubMed]
- Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009;101:621-7. [Crossref] [PubMed]
- Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. [Crossref] [PubMed]
- Bridgewater J, Lopes A, Palmer D, et al. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br J Cancer 2016;114:965-71. [Crossref] [PubMed]
- Subbiah IM, Subbiah V, Tsimberidou AM, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget 2013;4:156-65. [Crossref] [PubMed]
- Marcus L, Lemery SJ, Keegan P, et al. FDA Approval Summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res 2019;25:3753-8. [Crossref] [PubMed]
- Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 2018;29:1807-13. [Crossref] [PubMed]
- Ott PA, Piha-Paul SA, Munster P, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol 2017;28:1036-41. [Crossref] [PubMed]
- Ueno M, Chung HC, Nagrial A, et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol 2018;29:viii205-70.
- Ott PA, Bang YJ, Piha-Paul SA, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol 2019;37:318-27. [Crossref] [PubMed]
- Ninomiya K, Hotta K. Pembrolizumab for the first-line treatment of non-small cell lung cancer. Expert Opin Biol Ther 2018;18:1015-21. [Crossref] [PubMed]
- Deeks ED. Pembrolizumab: A Review in Advanced Melanoma. Drugs 2016;76:375-86. [Crossref] [PubMed]
- Mo H, Huang J, Xu J, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer 2018;119:538-45. [Crossref] [PubMed]