
Pyruvate kinase M2 is a marker of poor prognosis in lung adenocarcinoma but not lung squamous cell carcinoma
Introduction
Pyruvate kinases (PKs) facilitate the phosphorylation of adenosine diphosphate from phosphoenolpyruvate (PEP) during glycolysis to generate pyruvate and ATP (1). Mammalian PKs comprise four isoforms, namely, liver-type PK (PKL), red blood cell PK (PKR), and muscle isozymes Pyruvate Kinase M1 (PKM1) and Pyruvate Kinase M2 (PKM2) (2,3). PKM1 is found predominantly in skeletal muscles and in the brain, while PKM2 is universally expressed in proliferating cells and is a characteristic feature of tissues with a high nuclear formation rate. The position of PKM2 as the last irreversible step of the glycolytic pathway makes it a deciding factor for glycolytic flux and is hence known as the ‘‘glycolytic valve’’(4,5).
Several studies have demonstrated significant upregulation of PKM2 expression in many types of cancers, including lung cancer (6), colorectal cancer (7), and prostate cancer (8). Its upregulation is known to regulate aerobic glycolysis and promote the biosynthesis of cellular building blocks in tumor cells (9). Furthermore, its aberrant expression was also found to be associated with tumor progression, invasion, and metastasis (10). In lung cancer, inactivation of PKM2 enhanced mitochondrial respiration, suppressed the pentose phosphate pathway, and enhanced chemosensitivity (11). Additionally, knockdown of PKM2 in A549 cells was found to suppress cellular proliferation with restricted glucose uptake, ATP generation and fatty acid synthesis (12). Therefore, PKM2 has been considered a potential target for therapy against lung cancer.
Recent studies have shown that the elevated expression of PKM2 in lung adenocarcinoma (LUAD) is correlated with decreased overall and disease-free survival (13). Similar findings were also observed in oral squamous cell carcinoma (14), ovarian cancer (9), gallbladder cancer (15) and hepatocellular carcinoma (16). Non-small-cell lung cancer (NSCLC) is divided into three histological subtypes, lung squamous cell carcinoma (LUSC), LUAD, and large cell carcinoma, of which LUAD and LUSC are the major forms of NSCLC and have distinctly different molecular and clinical features (17). Previous studies showed that the same molecule exhibited different prognostic abilities in LUAD and LUSC. For example, although high expression of PD-L1 is linked to poor survival rate in patients with LUAD, no such associations were found in patients with LUSC (3). In contrast, elevated ASCL2 expression was a prognostic factor for improved overall survival in LUSC but not in LUAD (18).
Although many published papers have demonstrated that PKM2 might be a therapeutic target in NSCLC, its potential prognostic value in the subtypes of NSCLC has not been investigated. In this study, lung cancer data from the Cancer Genome Atlas (TCGA) were used to analyze the expression profile of PKM2 in LUAD and LUSC to assess its prognostic potential.
Methods
Retrospective data analysis in TCGA
This is a retrospective study using data from TCGA-LUAD and LUSC downloaded via the UCSC Xena browser (https://xenabrowser.net/). The project was approved by the 2016 National Natural Science Foundation of China - Henan Joint Fund Project for the screening of the effective components of Gualouxiebai Soup and the echanistic research of its anti-pulmonary fibrotic effects. RNA-seq was performed on 515 cancer tissues and 59 normal control tissues from TCGA-LUAD. Overall Survival (OS) data were obtained from 506 out of the 515 total patients. Similarly, RNA-seq data for TCGA-LUSC tumor tissues from 502 patients and normal tissues from 51 controls were used for study. Intact OS data were recorded for 495 patients. The clinicopathological information of patients with primary LUAD and LUSC, including age at diagnosis, sex, smoking history, nodal invasion, residual tumors, pathologic stage, recurrence status, Recurrence-Free Survival (RFS), Progression-Free Survival (PFS), living status and OS, were obtained for analysis.
Immunohistochemistry (IHC) evaluation of PKM2
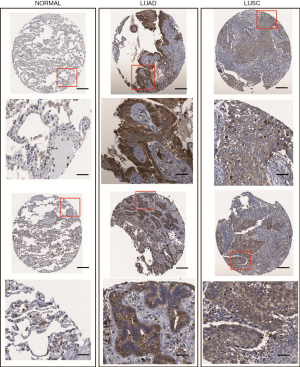
IHC stained images of PKM2 from the online Human Protein Atlas (www.proteinatlas.org/) (19,20) were used to compare normal alveolar epithelial tissues with LUAD or LUSC tissues.
Data mining in the Kaplan-Meier Plotter
PKM2 mRNA expression was monitored using the Kaplan-Meier Plotter (www.kmplot.com) online database (21), which has information on the gene expression and survival of 2,437 clinical lung cancer patients. Analysis of OS or PFS of patients with LUAD or LUSC was performed by splitting patient samples into two groups (high vs. low expression) followed by final assessment with a Kaplan-Meier survival plot, including hazard ratios (HRs) with 95% confidence intervals (CI) and log-rank P values.
Statistical analysis
GraphPad Prism 6.0 (GraphPad, Inc., CA, USA) and the SPSS 19.0 software package (SPSS, Inc., IL, USA) were used for statistical analysis. The expression of PKM2 was analyzed by Welch’s t-test in different groups. ROC curves were used to determine the Youden index for PKM2 expression (the best cutoff to separate patients), and then death and recurrence were analyzed by Kaplan-Meier curves of OS, RFS and PFS. PKM2 expression and the clinicopathological parameters were analyzed for any correlation by Chi-square tests and two-sided Fisher’s exact test. Log-rank tests were used to determine the significance of the difference between the curves. The prognostic significance of PKM2 was analyzed by univariate and multivariate Cox regression. In all the tests for statistical significance, P<0.05 was used as the threshold.
Results
PKM2 expression significantly increased in both LUAD and LUSC compared with normal tissues
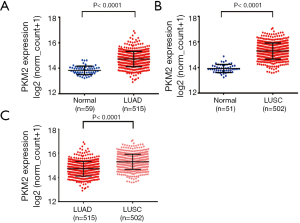
We compared PKM2 RNA expression in cancers with that in normal samples by using RNA-seq data from TCGA. The results showed that PKM2 expression was significantly increased in both LUAD (n=515) and LUSC (n=502) tissues compared with normal tissues (P<0.0001, Figure 1A,B). In addition, PKM2 expression in LUAD was markedly lower than that in LUSC (P<0.0001, Figure 1C). The IHC staining images from the Human Protein Atlas showed that normal respiratory tissues usually had low PKM2 staining (Figure 2, left). In comparison, LUAD and LUSC usually had moderate-to-strong PKM2 staining (Figure 2, middle and right).
PKM2 upregulation was associated with poor OS and RFS in patients with LUAD but not in patients with LUSC
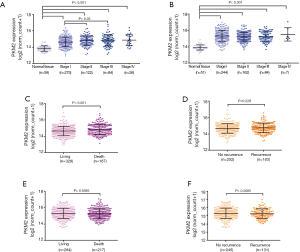
We further evaluated the expression profile of PKM2 in different pathologic stages in both LUAD and LUSC. The results showed that compared with normal tissues, PKM2 expression was obviously upregulated in stage I, stage II, stage III and stage IV in LUAD and LUSC (Figure 3A,B). In addition, stage II and stage III LUAD had significantly upregulated PKM2 expression compared with stage I LUAD tumors (Figure 3A). Then, we compared PKM2 expression in patients with different survival outcomes. For LUAD, we found that the deceased patients (n=187) had significantly higher PKM2 expression than the living patients (n=328; P<0.001, Figure 3C). However, no significant difference was observed between patients with recurrence (n=252) and patients without recurrence (n=183; P=0.2228, Figure 3D). In LUSC patients, these associations were not observed (Figure 3E,F).
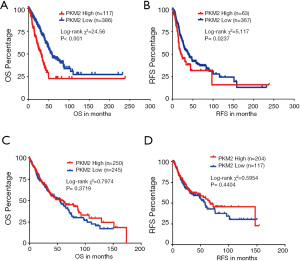
Kaplan-Meier survival curves were generated and analyzed to determine the association between the expression of PKM2 and the OS/RFS of patients with LUAD or LUSC. The patients were separated into high and low PKM2 expression groups based upon the best cutoff model. The LUAD patients with high PKM2 expression had shorter OS (P<0.001) and PFS (P<0.05) compared with that in the LUAD patients with low PKM2 expression (Figure 4A,B). However, these associations were not confirmed in LUSC patients (P=0.3719 for OS and 0.4404 for RFS, Figure 4C,D).
Association of increased PKM2 expression with the poor prognosis of patients with LUAD or LUSC
To explore the critical effect of PKM2 on the survival of patients with LUAD or LUSC, we further performed data mining in the Kaplan–Meier plotter (http://kmplot.com/analysis/index.php?p=service&cancer=lung). The Kaplan-Meier curve and log-rank test analyses revealed that the high PKM2 expression group had significantly inferior OS (HR: 2.56; 95% CI: 1.99–3.28; P<0.001; Figure 5A) and PFS (HR: 2.18; 95% CI: 1.58–3; P<0.001; Figure 5B). In comparison, there was no significant association between PKM2 expression and OS or PFS in patients with LUSC (HR: 1.13; 95% CI: 0.89–1.42; P=0.33 for OS; HR: 1.28; 95% CI: 0.77–2.15; P=0.34 for PFS; Figure 5C,D).
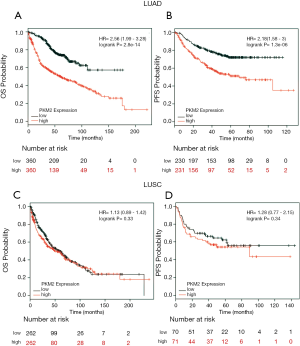
PKM2 expression was an independent prognostic factor in LUAD
Finally, the independent prognostic values of PKM2 expression in LUAD were verified. Table 1 represents the association between PKM2 expression and the different clinicopathological parameters of LUAD patients. Compared to low PKM2 expression, high PKM2 expression was more closely related to advanced stage (III/IV) (34/117 vs. 76/390; P=0.0303), nodal positive status (53/116 vs. 119/387; P=0.0037), and death (63/118 vs. 124/397; P<0.001), as revealed by Chi-square analysis.
Table 1
| Parameters | Classification | PKM2 expression | P value | |
|---|---|---|---|---|
| High (n=118) | Low (n=397) | |||
| Age (mean ± SE) | 65.11±0.9978 | 65.44±0.4976 | 0.7687 | |
| Gender | Female | 58 | 219 | 0.2930 |
| Male | 60 | 178 | ||
| Smoking history | 2/3/4/5 | 96 | 330 | 0.6551 |
| 1 | 19 | 56 | ||
| No data | 3 | 11 | ||
| Pathologic stage | III/IV | 34 | 76 | 0.0303 |
| I/II | 83 | 314 | ||
| No data | 1 | 7 | ||
| Nodal invasion | N0 | 63 | 268 | 0.0037 |
| N1/2/3 | 53 | 119 | ||
| Nx/no data | 2 | 0 | ||
| Residual tumors | R0 | 84 | 260 | 0.3870 |
| R1/R2 | 6 | 11 | ||
| Rx/no data | 28 | 126 | ||
| Recurrence status | No | 45 | 207 | 0.0736 |
| Yes | 46 | 137 | ||
| No data | 28 | 53 | ||
| Living status | Living | 55 | 273 | <0.0001 |
| Dead | 63 | 124 | ||
Smoking history: 1, lifelong nonsmoker; 2, current smoker; 3, former smoker (for >15 years); 4, former smoker (for ≤15 years); 5, former smoker (duration not specified); Nx, Regional lymph nodes could not be assessed. Rx, the presence of residual tumor could not be assessed; SE, standard error. PKM2, pyruvate kinase M2.
Univariate analysis revealed that poor OS of LUAD, in terms of advanced stage, positive nodal invasion and residual tumors, was directly associated with increased PKM2 expression (Table 2). Multivariate analysis (HR: 1.359; 95% CI: 1.009–1.829; P<0.05; Table 2) further validated that the increase in PKM2 expression can be used as an independent prognostic indicator of poor OS.
Table 2
| Parameters | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| Age | 0.332 | 1.008 | 0.992–1.023 | ||||
| Gender: female vs. male | 0.672 | 1.065 | 0.796–1.424 | ||||
| Smoking history: 1 vs. 2/3/4/5 | 0.682 | 1.090 | 0.722–1.645 | ||||
| Pathological state: I/II vs. III/IV | 0.000 | 0.375 | 0.275–0.511 | 0.008 | 0.567 | 0.374–0.860 | |
| Nodal status: negative vs. positive | 0.000 | 0.389 | 0.289–0.523 | 0.006 | 0.578 | 0.391–0.853 | |
| Residual tumors: no vs. yes | 0.000 | 0.252 | 0.140–0.451 | 0.001 | 0.361 | 0.195–0.671 | |
| PKM2 expression | 0.000 | 1.626 | 1.266–2.087 | 0.043 | 1.359 | 1.009–1.829 | |
PKM2, pyruvate kinase M2; HR, hazard ratio; CI, confidence interval.
Discussion
Lung cancer is grouped as small-cell lung cancer (SCLC) and NSCLC depending upon the histological characteristics. LUAD and LUSC are the most common types, comprising 60% and 25% of all NSCLC cases, respectively (22). Cumulative studies indicate that the molecular mechanisms of carcinogenesis in LUAD and LUSC are highly variable, and hence, most therapeutic strategies for LUAD are ineffective against LUSC (23-26). An increasing number of studies have demonstrated that an individual molecule might play different regulatory roles in cancer cell behaviors and may have different prognostic value in these two subtypes. For example, elevated expression of survivin was found in LUAD as well as LUSC, but higher survivin expression was only correlated with worse survival outcomes of LUAD (27). In contrast, Polo-like kinase 1 (PLK1) was found to be significantly implicated in the prognosis of LUSC but not LUAD (28). Other studies have indicated that while overexpression of SOX2 is linked to better survival outcome in LUSC (25), but its association with LUAD points to unfavorable survival and an increase in cancer stem cell properties (29,30). Hence, the prognostic value of PKM2 in these two subtypes of NSCLC was evaluated.
In the present study, using data from TCGA-LUAD/LUSC, we verified that PKM2 mRNA and protein expression levels were significantly elevated in both LUAD and LUSC. PKM2 expression in LUSC was comparatively higher than that in LUAD tissues. Interestingly, data on the 10-year survival of 506 patients with LUAD and 495 patients with LUSC confirmed the association of poor OS and RFS with an increase in the expression of PKM2 in LUAD but not in LUSC. With an HR of 1.359 (95% CI: 1.009–1.829; P<0.05), multivariate and univariate analyses revealed that the increase in PKM2 can be exploited as an independent prognostic indicator of poor OS in LUAD. The Kaplan-Meier plotter was used to further validate that PKM2 upregulation is a predictor of poor prognosis in patients with LUAD. However, no such associations were found in patients with LUSC. The results prompted us to speculate that PKM2 upregulation is an important prognostic biomarker in LUAD but not in LUSC. This difference might be explained by the different energy metabolism between LUAD and LUSC. LUAD mainly relies on aerobic glycolysis for ATP generation (31). In contrast to LUAD, LUSC acquires ATPs in a more complex manner; it dominantly utilizes mitochondrial oxidation under normal oxygen levels, but under hypoxic conditions, both mitochondrial oxidation and anaerobic glycolysis pathways are used as energy sources (32). Notably, when cancer cells are genetically engineered to replace PKM2 with PKM1, the cells switch to mitochondrial respiration from aerobic glycolysis and lose the ability to form tumors following xenografting (33).
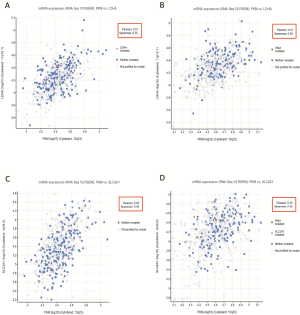
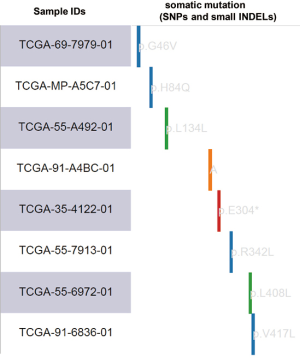
Glucose transporter-1 (encoded by the SLC2A1 gene) and lactate dehydrogenase-A (LDHA) are two important regulators associated with the Warburg effect (32,34). SLC2A1 and LDHA upregulation have been verified as independent prognostic indicators of poor survival in LUAD but not in LUSC (35). To confirm the metabolic difference between LUAD and LUSC, we further evaluated the coexpression of SLC2A1/LDHA and PKM2 in LUAD and LUSC based on RNA-seq data in TCGA-LUAD. Correlation analysis demonstrated a moderate positive correlation between LDHA and PKM2 in LUAD (Pearson’s r=0.51; Figure S1A) but only revealed a weak positive correlation in LUSC (Pearson’s r=0.41; Figure S1B). There was also a moderate positive correlation between SLC2A1 and PKM2 in LUAD (Pearson’s r=0.50; Figure S1C) but only a weak positive correlation in LUSC (Pearson’s r=0.35; Figure S1D). These findings suggest that different energy metabolism dynamics exists in LUAD and LUSC. By using the SNP and small INDEL data in TCGA-LUAD, we further assessed the association between PKM2 expression and its DNA mutations. Among 488 LUAD patients with available PKM2 expression and mutation data, we found that eight cases had SNPs/small INDELs (Figure S2).
Altered metabolism to generate the nutrients required for proliferation is a signature marker of cancer cells. Overexpression of PKM2 has been reported in several types of cancerous tissues, including those from the lung, breast, prostate, blood, cervix, kidney, bladder, papillary thyroid and colon (35). The distinctive dimerization tendency of PKM2 inside cancer cells increases its efficiency for anabolic glycolysis, contributing to enhanced macromolecular biosynthesis for vigorous cell proliferation (36,37). Therefore, since glycolysis highly relies on PKM2, it is reasonable to infer that PKM2 upregulation might increase the aerobic glycolysis rate, which facilitates cancer cell proliferation, invasion, and even metastasis in LUAD. Consistent with this judgment, several recent studies found that inhibition of PKM2 significantly decreased pyruvate kinase activity and glycolysis, reduced the cellular ATP level and promoted mitochondrial biogenesis, autophagy, and cancer cell survival in LUAD (38,39). Moreover, knockdown of PKM2 significantly inhibited tumor growth and invasion both in vitro and in patients with LUAD (12). However, in the future, more studies are needed to investigate the association between PKM2 expression and glycolysis in LUSC.
Conclusions
Despite the significantly upregulated expression of PKM2 in both LUAD and LUSC, its potential as an independent prognostic indicator of poor OS and RFS is applicable for LUAD but not for LUSC.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The project was approved by the 2016 National Natural Science Foundation of China - Henan Joint Fund Project for the screening of the effective components of Gualouxiebai Soup and the mechanistic research of its anti-pulmonary fibrotic effects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Genetic research in psychiatry. Proceedings of a "Munchener Genetik-Gesprache" International Symposium. Berlin, 27-28 September 1986. A satellite symposium of the VIIth International Congress on Human Genetics. J Psychiatr Res 1987;21:333-647. [PubMed]
- Bian Z, Zhang J, Li M, et al. LncRNA-FEZF1-AS1 Promotes Tumor Proliferation and Metastasis in Colorectal Cancer by Regulating PKM2 Signaling. Clin Cancer Res 2018;24:4808-19. [Crossref] [PubMed]
- Poliakov E, Managadze D, Rogozin IB. Generalized portrait of cancer metabolic pathways inferred from a list of genes overexpressed in cancer. Genet Res Int 2014;2014:646193.
- Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Chang JT, Lee YM, Huang RS. The impact of the Cancer Genome Atlas on lung cancer. Transl Res 2015;166:568-85. [Crossref] [PubMed]
- Chao TK, Huang TS, Liao YP, et al. Pyruvate kinase M2 is a poor prognostic marker of and a therapeutic target in ovarian cancer. PLoS One 2017;12:e0182166. [Crossref] [PubMed]
- Xie Y, Cao H, Zhang Z, et al. Molecular network of miR-1343 regulates the pluripotency of porcine pluripotent stem cells via repressing OTX2 expression. RNA Biol 2019;16:82-92. [Crossref] [PubMed]
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008;452:230-3. [Crossref] [PubMed]
- Chu B, Wang J, Wang Y, et al. Knockdown of PKM2 induces apoptosis and autophagy in human A549 alveolar adenocarcinoma cells. Mol Med Rep 2015;12:4358-63. [Crossref] [PubMed]
- Gui DY, Lewis CA, Vander Heiden MG. Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci Signal 2013;6:pe7. [Crossref] [PubMed]
- Snaebjornsson MT, Schulze A. Non-canonical functions of enzymes facilitate cross-talk between cell metabolic and regulatory pathways. Exp Mol Med 2018;50:34. [Crossref] [PubMed]
- Guo CY, Yan C, Luo L, et al. Enhanced expression of PKM2 associates with the biological properties of cancer stem cells from A549 human lung cancer cells. Oncol Rep 2017;37:2161-6. [Crossref] [PubMed]
- Győrffy B, Surowiak P, Budczies J, et al. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One 2013;8:e82241. [Crossref] [PubMed]
- Hu W, Lu SX, Li M, et al. Pyruvate kinase M2 prevents apoptosis via modulating Bim stability and associates with poor outcome in hepatocellular carcinoma. Oncotarget 2015;6:6570-83. [Crossref] [PubMed]
- Hu XG, Chen L, Wang QL, et al. Elevated expression of ASCL2 is an independent prognostic indicator in lung squamous cell carcinoma. J Clin Pathol 2016;69:313-8. [Crossref] [PubMed]
- Huang P, Zhao X, Xiao W, et al. 18F-fluorodeoxyglucose uptake predicts PKM2 expression in lung adenocarcinoma. Oncotarget 2017;8:39618-26. [Crossref] [PubMed]
- Koh YW, Lee SJ, Park SY. Differential expression and prognostic significance of GLUT1 according to histologic type of non-small-cell lung cancer and its association with volume-dependent parameters. Lung Cancer 2017;104:31-7. [Crossref] [PubMed]
- Li C, Zhao Z, Zhou Z, et al. PKM2 Promotes Cell Survival and Invasion Under Metabolic Stress by Enhancing Warburg Effect in Pancreatic Ductal Adenocarcinoma. Dig Dis Sci 2016;61:767-73. [Crossref] [PubMed]
- Li H, Wang H, Sun Z, et al. The clinical and prognostic value of polo-like kinase 1 in lung squamous cell carcinoma patients: immunohistochemical analysis. Biosci Rep 2017; [Crossref] [PubMed]
- Lu W, Cao Y, Zhang Y, et al. Up-regulation of PKM2 promote malignancy and related to adverse prognostic risk factor in human gallbladder cancer. Sci Rep 2016;6:26351. [Crossref] [PubMed]
- Wei Y, Wang D, Jin F, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun 2017;8:14041. [Crossref] [PubMed]
- Meijer TW, Schuurbiers OC, Kaanders JH, et al. Differences in metabolism between adeno- and squamous cell non-small cell lung carcinomas: spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer 2012;76:316-23. [Crossref] [PubMed]
- Miao P, Sheng S, Sun X, et al. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life 2013;65:904-10. [Crossref] [PubMed]
- Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem 1986;261:13807-12. [PubMed]
- Noguchi T, Yamada K, Inoue H, et al. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem 1987;262:14366-71. [PubMed]
- Prakasam G, Singh RK, Iqbal MA, et al. Pyruvate kinase M knockdown-induced signaling via AMP-activated protein kinase promotes mitochondrial biogenesis, autophagy, and cancer cell survival. J Biol Chem 2017;292:15561-76. [Crossref] [PubMed]
- Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 2012;18:1167-76. [Crossref] [PubMed]
- Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer 2016;98:69-75. [Crossref] [PubMed]
- Schuurbiers OC, Meijer TW, Kaanders JH, et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J Thorac Oncol 2014;9:1485-93. [Crossref] [PubMed]
- Sun H, Zhu A, Zhang L, et al. Knockdown of PKM2 Suppresses Tumor Growth and Invasion in Lung Adenocarcinoma. Int J Mol Sci 2015;16:24574-87. [Crossref] [PubMed]
- Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem 2014;289:7884-96. [Crossref] [PubMed]
- Sun PL, Jin Y, Kim H, et al. Survivin expression is an independent poor prognostic marker in lung adenocarcinoma but not in squamous cell carcinoma. Virchows Arch 2013;463:427-36. [Crossref] [PubMed]
- Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [Crossref] [PubMed]
- Wang X, Xu Y, Jiang C, et al. LincRNA-p21 suppresses development of human prostate cancer through inhibition of PKM2. Cell Prolif 2017; [Crossref] [PubMed]
- Yan XL, Zhang XB, Ao R, et al. Effects of shRNA-Mediated Silencing of PKM2 Gene on Aerobic Glycolysis, Cell Migration, Cell Invasion, and Apoptosis in Colorectal Cancer Cells. J Cell Biochem 2017;118:4792-803. [Crossref] [PubMed]
- Yang YC, Cheng TY, Huang SM, et al. Cytosolic PKM2 stabilizes mutant EGFR protein expression through regulating HSP90-EGFR association. Oncogene 2016;35:3387-98. [Crossref] [PubMed]
- Yu C, Hou L, Cui H, et al. LDHA upregulation independently predicts poor survival in lung adenocarcinoma, but not in lung squamous cell carcinoma. Future Oncol 2018;14:2483-92. [Crossref] [PubMed]
- Yu Z, Huang L, Qiao P, et al. PKM2 Thr454 phosphorylation increases its nuclear translocation and promotes xenograft tumor growth in A549 human lung cancer cells. Biochem Biophys Res Commun 2016;473:953-8. [Crossref] [PubMed]


