
Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report and literature review
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor with significant differences in regional distribution, which has a high incidence in Southeast Asia, North Africa, and the Arctic regions, especially in southern China (1). In the 4th edition of the World Health Organization (WHO) classifications of head and neck tumors in 2017, NPC was classified into the following subtypes: non-keratinizing squamous cell carcinoma, keratinizing squamous cell carcinoma, basaloid squamous cell carcinoma, and nasopharyngeal papillary adenocarcinoma (NPPA) (2). NPPA includes conventional or mucosal surface origin type and the salivary gland type, and thyroid-like low-grade NPPA (TL-LGNPPA) belongs to the former one (3).
TL-LGNPPA, a rare neoplasm originating from the nasopharyngeal epithelium, accounting for 0.38–0.48% of all malignant neoplasms in the nasopharynx (4,5), which was first defined by Carrizo et al. in 2005 (6). TL-LGNPPA is a low-grade malignancy, which has similar pathological and immunohistochemical findings compared to papillary thyroid carcinoma. The TL-LGNPPA is characterized by a linear arrangement of bland cuboidal to columnar cells with papillary lobes and dense glandular cells (3,4,7). In addition, overexpression of thyroid transcription factor 1 (TTF-1) was found in the majority of TL-LGNPPA patients (6,8).
Owing to the rarity of this entity, the clinicopathological characteristics, treatment modalities, and prognosis of TL-LGNPPA have not been well described. We reported a case of TL-LGNPPA in a 42-year-old female. Another purpose was to present the literature review of TL-LGNPPA by focusing on clinicopathological, immunohistochemical features, clinical management, and prognosis of this rare tumor. We presented the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-1973).
Case presentation
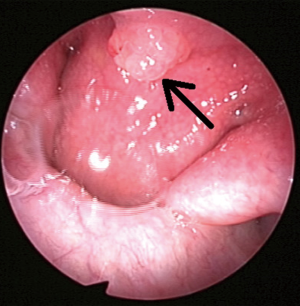
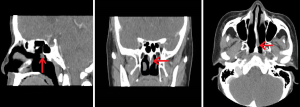
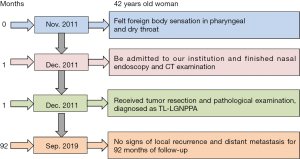
A 42 years old non-smoking Chinese woman was admitted to our institution because of foreign body sensation in pharyngeal and dry throat for one month on December, 28, 2011. Nasal endoscopy showed a gray-red mass with a smooth surface at the junction of the nasopharyngeal wall and free margin of the nasal septum, which measured as 0.5 cm × 0.5 cm (Figure 1). A contrast-enhanced computed tomography (CT) was conducted on this patient, and the CT images showed a soft tissue at the junction of the nasopharyngeal wall and free margin of the nasal septum with no signs of local infiltration and bone erosion (Figure 2). In addition, there was no lymphadenopathy in the cervix and no lesion in the thyroid gland. Moreover, no family history of cancer was found.
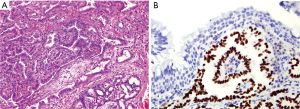
The patient received surgical treatment and achieved complete excision on December 29, 2011. The tumor tissues were fixed with 4% formaldehyde, then dehydrated and embedded with paraffin, followed-by sectioned and stained with hematoxylin-eosin. Finally, a two-step EnVision method was used for the detection of TTF-1 expression. Microscopically, the tumor was present with papillary structure and dense glandular cells (Figure 3A). In addition, the tumor cells exhibited a diffuse expression of TTF-1 (Figure 3B). According to these findings, a diagnosis of TL-LGNPPA was started. No adjuvant chemotherapy or radiotherapy was performed following surgery. This patient was a follow-up for 93 months with no signs of local recurrence and distant metastasis from December 29, 2011, to September 20, 2019. No unanticipated events after surgery were reported in this patient. The timeline of the symptoms, interventions, and outcome of this case is listed in Figure 4. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Xiamen University, and written informed consent was obtained from the patient for publication of this case report and any accompanying images (Approval number. KYH2019-044).
Search strategy for literature review
We conducted a comprehensive review of the current literature using the database from PubMed, Science Direct, Web of Science, Google Scholar, and China National Knowledge Internet up to September 26, 2019. The following keywords were used as effective index words: “nasopharyngeal papillary adenocarcinoma or low-grade nasopharyngeal papillary adenocarcinoma or thyroid-like nasopharyngeal papillary adenocarcinoma or thyroid-like low-grade nasopharyngeal papillary adenocarcinoma.” The literature with “thyroid-like low-grade nasopharyngeal papillary adenocarcinoma” were identified, and we excluded patients diagnosed with nasopharyngeal adenocarcinoma, nasopharyngeal papillary adenocarcinoma, and low-grade nasopharyngeal papillary adenocarcinoma. Thirty-four literatures were included from 2005 to 2019 (4-6,8-16) (Table S1).
Patient characteristics from the literature review
A total of 46 patients, including one from our institution were identified in the 34 literature, including 24 English literature and 10 Chinese literature. The patients’ characteristics have shown in Table S1. The median age of diagnosis was 34 years (range, 9–68 years), and there was no sex predilection (males: females = 1:1.19). The distribution of main countries of the published literature was as follows: China (60.9%), Japan (10.8%), United States (8.7%), Argentina (4.3%), Turkey (4.3%), Vietnam (2.2%), Iran (2.2%), Spain (2.2%), United Kingdom (2.2%), and Italy (2.2%). The most common clinical manifestations were nasal obstruction (45.6%), epistaxis and bloody sputum (32.6%), foreign body sensation of the nasal cavity, nasopharynx, and pharynx (19.6%). In addition, 10.9% of the patients were diagnosed during the physical examination, and 8.7% of patients performed as snoring, sore throat, or headache. Imaging examination [CT, magnetic resonance imaging (MRI), or endoscope] found that most tumors originated from the unilateral or bilateral posterior roof of the nasopharynx (76.1%), and the others occurred in the posterior edge of the nasal septum (23.9%).
Immunohistochemistry findings, treatment, and prognosis
All patients received an excisional biopsy and histopathologic evaluation. In patients with available immunohistochemical results (Figure 5), all patients were overexpression of thyroid transcription factor 1 (100%), CK7 (100%), CK19 (100%), Ckpan (100%), and epithelial membrane antigen (EMA) (100%). In addition, the majority of them were overexpression of Vimentin (94.7%). However, TL-LGNPPA patients were more likely to have lower-expression of CK20 (24/24, 100%), smooth muscle actin (SMA) (7/7, 100%), Epstein-Barr virus-encoded RNA (EBER) (24/24, 100%), thyroglobulin (TG) (44/45, 97.8%), CK5/6 (25/27, 92.6%), S-100 (26/30, 86.7%), and P63 (12/14, 85.8%) for this population. All patients received surgical treatment, and only two patients (4.3%) received postoperative radiotherapy (the radiotherapy dose was not mentioned in the literature). No other adjuvant treatment strategies were found in all literature. With a median follow-up of 16 months (range, 3–240 months) (n=39), and 16% of them were follow-up for more than 5 years, no locoregional recurrences or distant metastasizes occurred.
Discussion
In this study, we reported one case of TL-LGNPPA and performed a comprehensive literature review to analyze the clinicopathological features, clinical management, and prognosis of TL-LGNPPA. Our results showed that TL-LGNPPA was a rare disease with unique clinical and immunohistochemical features, and excellent prognosis.
Different from the common histology of NPC, the pathogenesis and etiology of TL-LGNPPAs remain to be elucidated. This literature review found that most patients were Asian (76.1%). Currently, no definitive risk factors, such as genetic predisposition, environmental, and radiation exposure, have been found to contribute to the development of the TL-LGNPPA (5). Epstein-Barr virus (EBV) has been confirmed to be associated with the development of NPC, especially in non-keratinizing histology (17). However, all patients with available EBER status were negative in our literature review. Therefore, the EBV status might not contribute to the development and progression of TL-LGNPPA. More studies are required to elaborate on the pathogenesis and etiology of this rare disease.
The diagnosis of TL-LGNPPA mainly based on the unique pathological and immunohistochemical features, including TTF-1 positive and TG negative. It should be distinguished with nasopharyngeal metastatic papillary thyroid carcinoma due to their similar pathological characteristics and similar expression of TTF-1 (9). TG was a useful biomarker to differentiate these two diseases. Lower expression of TG was found in TL-LGNPPA, and overexpression of TG was observed in nasopharyngeal metastatic papillary thyroid (11,18). TG is a secretory protein produced by vertebrate, which is synthesized in the thyroid cell, and stained positive in papillary thyroid carcinoma (8,19). In this literature review, all of the 46 patients stained positive in TTF-1, and of the 45 patients known the expression of TG, only one (2.2%) patient was positive for TG (16). Therefore, the expression of TG was also an important biomarker for the diagnosis of TL-LGNPPA.
It is also challenging to distinguish TL-LGNPPA from low-grade papillary adenocarcinoma of the salivary gland. Salivary gland types exclusively occur in the palate, but occasionally in the nasopharynx, which originate minor salivary glands. Almost all of them were found in older patients with more aggressive behaviors, including a higher risk of local recurrence and lymph node metastasis (20,21). However, the median age at onset of TL-LGNPPA in our study was 34 years, which was significantly younger than patients with low-grade papillary adenocarcinoma of the salivary gland, and was also significantly younger compared to common histology of NPC (median age at onset, 59 years) (22). In addition, salivary gland types showed overexpression of S-100, while TL-LGNPPAs were more likely to be lower expression of S-100 (5). Moreover, salivary gland types had an inferior outcome and required adjuvant systemic therapy, which was significantly different from TL-LGNPPAs that were surgically curable (21).
In our literature review, we found that CK7, CK19, Ckpan, EMA, and Vimentin were also overexpression in this rare disease. Therefore, these markers could also help to diagnose the TL-LGNPPA. In National Comprehensive Cancer Network guidelines, the common histological subtypes of NPC are mainly treated by radiotherapy and chemotherapy, and surgery is recommended in specific circumstances (23). In the imaging findings of TL-LGNPPA, the tumors exhibited exogenous growth without local invasion and lymph node metastasis, which was significantly different from the common histological subtypes of NPC (24). All patients in our literature review received surgical treatment, no locoregional recurrences or distant metastasizes occurred during the follow-up time. The woman in our institution also survived with no signs of local or distant recurrence after 93 months of follow-up. Therefore, surgery is the optimal treatment for this patient subset. In this literature review, two of them in our literature review received postoperative radiotherapy (8). The reasons for postoperative radiotherapy in these two patients were not described in the literature. Based on our findings, radiotherapy should be omitted for TL-LGNPPA.
To the best of our knowledge, our study had the largest sample size, including one patient from our institution and 45 patients from the literature review to assess the clinicopathological features, treatment, and prognosis of TL-LGNPPA.
Several limitations should be noted in this study. Firstly, there might be a selection bias that only English and Chinese literature were included. Secondly, among all biomarkers, only TTF-1 was examined due to the financial constraint of the patient at that time.
Conclusions
In conclusion, our study suggests that TL-LGNPPA is an extremely rare entity with unique disease features and excellent prognosis. Surgery is the optimal treatment for this population.
Table S1
| Author (reference) | Age (years) | Gender | Country | Symptoms | Tumor size | Treatment | Follow-up time |
|---|---|---|---|---|---|---|---|
| References in the main test | |||||||
| Li et al. (4) | 35 | F | China | Dyspnea, nasal foreign body sensation, dry throat | 1.5 cm × 1 cm × 0.8 cm | Surgery | 16 months |
| Zhang et al. (5) | 64 | M | China | Nasal bleeding, nasal foreign body sensation | 2 cm | Surgery | 1 year |
| Carrizo et al. (6) | 9 | M | Argentina | Right nasal fullness, blood in the saliva | 2.0 cm | Surgery | 2 years |
| 13 | M | Argentina | Unilateral nasal obstruction | 1.5 cm | Surgery | 15 years | |
| Huang et al. (8) | 26 | F | China | Nasal obstruction, epistaxis, occasional rhinorrhea | 1.5 cm | Surgery | 8.8 years |
| 44 | M | China | No associated symptoms | 0.4 cm | Surgery + radiotherapy | 5.5 years | |
| 19 | M | China | Nasal obstruction, epistaxis, occasional rhinorrhea | 1.0 cm | Surgery | 1.5 years | |
| 29 | M | China | Pharyngeal discomfort | 1.0 cm | Surgery + radiotherapy | 7 months | |
| 36 | F | China | Detection in physical examination | 1.2 cm | Surgery | 6 months | |
| Li et al. (9) | 15 | F | China | Rhinorrhea, nasal congestion | 2.5 cm × 2 cm | Surgery | 2 years |
| Yang et al. (10) | 27 | F | China | Blocked nose, rhinorrhea, mild headache | 2.1 cm × 1.8 cm | Surgery | 3 years |
| 34 | F | China | Tinnitus and loss of hearing | 0.5 cm | Surgery | 12 months | |
| 23 | M | China | Nasal discomfort | 0.5 cm | Surgery | 12 months | |
| Baumann et al. (11) | 26 | M | United States | Headaches, nasal congestion, epistaxis | 0.8 cm | Surgery | NA |
| Borsetto et al. (12) | 15 | F | Italy | Posterior nose bleeding | 1.29 cm3 | Surgery | 30 months |
| Sourati et al. (13) | 35 | F | Iran | Nasal obstruction with mild post-nasal drip | 2 cm × 2 cm × 1 cm | Surgery | 6 years |
| Le et al. (14) | 50 | F | Vietnam | Facial pain | 4 cm | Surgery | NA |
| Oide et al. (15) | 68 | M | Japan | Sore throat, hemosputum | 0.8 cm × 0.4 cm | Surgery | NA |
| Ozer et al. (16) | 17 | F | Turkey | Nasal obstruction | 2.7 cm × 2.6 cm | Surgery | 12 months |
| Present study | 42 | F | China | Pharyngeal foreign body sensation | 0.5 cm × 0.5 cm × 0.5 cm | Surgery | 7.7 years |
| References in the supplementary material | |||||||
| Pineda-Daboin et al. (25) | 9 | M | United States | Nasal obstruction | NA | Surgery | 5 years |
| 13 | M | United States | Nasal obstruction | NA | Surgery | 20 years | |
| Ohe et al. (26) | 25 | M | Japan | Bloody sputum | 0.8 cm | Surgery | 13 months |
| 41 | M | Japan | No associated symptoms | 0.5 cm | Surgery | 9 months | |
| Li et al. (27) | 26 | F | China | Nasal obstruction, bloody sputum | 1.5 cm × 1.3 cm × 1.4 cm | Surgery | 8 months |
| Wu et al. (28) | 36 | F | China | No associated symptoms | NA | Surgery | 3 years |
| Du et al. (29) | 47 | F | China | Bloody sputum, nasal foreign body sensation | 1 cm × 0.8 cm × 0.6 cm | Surgery | 3 years |
| Horino et al. (30) | 25 | F | Japan | Fever of unknown origin | 1.7 cm × 1.2 cm | Surgery | 3 years |
| Zhou et al. (31) | 49 | M | China | Nasal obstruction, bloody sputum | 2.5 cm × 1.5 cm × 1.5 cm | Surgery | 3 months |
| Oishi et al. (32) | 47 | F | Japan | Nasal obstruction | 2 cm | Surgery | 19 months |
| Niu et al. (33) | 43 | F | China | Neck discomfort | 1.2 cm × 1.2 cm × 1.2 cm | Surgery | 8 months |
| Fu et al. (34) | 68 | M | China | Pharyngeal foreign body sensation | NA | Surgery | 12 months |
| Sillings et al. (35) | 19 | M | United States | Epistaxis, nasal congestion | 1.5 cm | Surgery | NA |
| Appukutty et al. (36) | 49 | M | Britain | Snoring | 0.6 cm × 0.6 cm × 0.5 cm | Surgery | 9 months |
| Ozturk et al. (37) | 24 | F | Turkey | Nasal congestion | 3 cm × 2.5 cm | Surgery | 4 years |
| Petersson et al. (38) | 39 | F | China | Epistaxis, blocked nose, rhinorrhea | 1 cm | Surgery | NA |
| Liu et al. (39) | 38 | F | China | Nasal foreign body sensation | 1 cm × 0.7 cm × 0.5 cm | Surgery | 15 months |
| Yao et al. (40) | 44 | F | China | Nasal obstruction, blood in the sputum | 1.2 cm × 1 cm × 0.4 cm | Surgery | 14 months |
| 67 | M | China | Hoarseness, pharyngeal foreign body sensation | 0.8 cm × 0.4 cm × 0.2 cm | Surgery | NA | |
| Zhang et al. (41) | 16 | F | China | Nasal obstruction, rhinorrhea | 1.5 cm × 1 cm × 0.5 cm | Surgery | NA |
| Dai et al. (42) | 31 | F | China | Bloody sputum | 1.5 cm × 1 cm × 0.5 cm | Surgery | 16 months |
| Liu et al. (43) | 25 | M | China | Nasal congestion | 1 cm × 1 cm | Surgery | 19 months |
| 57 | M | China | Nasal congestion, dizziness | 1.2 cm × 0.8 cm × 0.8 cm | Surgery | 4 months | |
| Tang et al. (44) | 22 | M | China | Epistaxis, nasal congestion, bloody sputum | 1.5 cm × 0.9 cm × 0.9 cm | Surgery | 1 year |
| García-Gómez et al. (45) | 40 | F | Spain | Right ear serous otitis media | 2 cm × 2 cm × 1.7 cm | Surgery | 1 year |
| Chen et al. (46) | 34 | F | China | Detection in physical examination | 0.5 cm × 0.3 cm | Surgery | 3.5 years |
F, female; M, male; NA, not available.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1973). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Xiamen University, and written informed consent was obtained from the patient for publication of this case report and any accompanying images (Approval number. KYH2019-044).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- El-Naggar AK, Chan JKC, Grandis JR. WHO classification of tumors of head and neck tumours. 4 th ed. Lyon: IARC Press, 2017:1-347.
- Kakkar A, Sakthivel P, Mahajan S, et al. Nasopharyngeal Papillary Adenocarcinoma as a Second Head and Neck Malignancy. Head Neck Pathol 2019;13:699-704. [Crossref] [PubMed]
- Li M, Wei J, Yao X, et al. Clinicopathological Features of Low-Grade Thyroid-like Nasopharyngeal Papillary Adenocarcinoma. Cancer Res Treat 2017;49:213-8. [Crossref] [PubMed]
- Zhang WL, Ma S, Havrilla L, et al. Primary thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: A case report and literature review. Medicine (Baltimore) 2017;96:e8851. [Crossref] [PubMed]
- Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol 2005;9:189-92. [Crossref] [PubMed]
- Wang X, Yan H, Luo Y, et al. Low-grade nasopharyngeal papillary adenocarcinoma: a case report and review of the literature. Onco Targets Ther 2016;9:2955-9. [PubMed]
- Huang F, Xiang X, Hong B, et al. Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma. Am J Clin Pathol 2019;152:582-9. [Crossref] [PubMed]
- Li L, Zhou F, Lin F, et al. Clinicopathologic Characteristics of Thyroid-like Low-grade Nasopharyngeal Papillary Adenocarcinoma: A Case Report. Appl Immunohistochem Mol Morphol 2019;27:e81-4. [Crossref] [PubMed]
- Yang SC, Huang Y, Lu XF, et al. Thyroid-like low-grade nasopharyngeal papillary carcinoma with a “biphasic” morphology: report of 3 cases and literature review. Int J Clin Exp Pathol 2017;10:6038-46.
- Baumann KB, Betz SJ. Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma. Head Neck Pathol 2019;13:661-3. [Crossref] [PubMed]
- Borsetto D, Cazzador D, Prosenikliev V, et al. Nasopharyngeal thyroid-like low-grade papillary adenocarcinoma. B-ENT 2016;12:235-40. [PubMed]
- Sourati A, Malekzadeh M, Rakhshan A. Thyroid-like low-grade papillary adenocarcinoma of nasopharynx. BMJ Case Rep 2019;12:e226949. [Crossref] [PubMed]
- Le QV, Ngo DQ, Tran TD, et al. Primary Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma. Ear Nose Throat J 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Oide T, Kadosono O, Matsushima J, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with squamous differentiation: a novel histological finding. Hum Pathol 2017;70:43-8. [Crossref] [PubMed]
- Ozer S, Kayahan B, Cabbarzade C, et al. Thyroid-like papillary adenocarcinoma of the nasopharynx with focal thyroglobulin expression. Pathology 2013;45:622-4. [Crossref] [PubMed]
- Tsao SW, Tsang CM, To KF, et al. The role of Epstein-Barr virus in epithelial malignancies. J Pathol 2015;235:323-33. [Crossref] [PubMed]
- Prpić M, Franceschi M, Romić M, et al. Thyroglobulin as a tumor marker in differentiated thyroid cancer - clinical considerations. Acta Clin Croat 2018;57:518-27. [PubMed]
- Di Jeso B, Arvan P. Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr Rev 2016;37:2-36. [Crossref] [PubMed]
- Kuan EC, Alonso JE, Arshi A, et al. Nasopharyngeal adenocarcinoma: a population-based analysis. Am J Otolaryngol 2017;38:297-300. [Crossref] [PubMed]
- Liu LZ, Zhang YM, Chen Y, et al. Spreading patterns, prognostic factors and treatment outcomes of nasopharyngeal papillary adenocarcinoma and salivary gland-type carcinomas. Clin Otolaryngol 2016;41:160-8. [Crossref] [PubMed]
- Booth JR, Unsal AA, Tadros S, et al. Salivary Gland Cancers of the Nasopharynx: A Population-Based Analysis of 383 Cases. Otolaryngol Head Neck Surg 2019;161:442-9. [Crossref] [PubMed]
- NCCN. NCCN clinical Practice guidelines in oncology V.2.2019. Head and Neck Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- Xu Y, Huang T, Fan L,et al. Patterns and Prognostic Value of Lymph Node Metastasis on Distant Metastasis and Survival in Nasopharyngeal Carcinoma: A Surveillance, Epidemiology, and End Results Study, 2006-2015. J Oncol 2019;2019:4094395.
- Pineda-Daboin K, Neto A, Ochoa-Perez V, et al. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol 2006;10:215-21. [Crossref] [PubMed]
- Ohe C, Sakaida N, Tadokoro C, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int 2010;60:107-11. [Crossref] [PubMed]
- Li JF, Ye Q, Hong B, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of a case. Zhonghua Bing Li Xue Za Zhi 2011;40:638-9. [PubMed]
- Wu PY, Huang CC, Chen HK, et al. Adult thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol Head Neck Surg 2007;137:837-8. [Crossref] [PubMed]
- Du W, Zhou Q, Yang LM, et al. Primary thyroid-like low-grade papillary adenocarcinoma of the nasopharynx: a clinicopathological analysis. J Diag pathol 2016;23:684-8.
- Horino T, Ichii O, Hamada-Ode K, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: A case report. Mol Clin Oncol 2016;5:693-6. [Crossref] [PubMed]
- Zhou YH, Liu XJ, Wang J, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with papillary carcinoma of thyroid: report of a case. Zhonghua Bing Li Xue Za Zhi 2016;45:801-2. [PubMed]
- Oishi N, Kondo T, Nakazawa T, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma:Case report and literature review. Pathol Res Pract 2014;210:1142-5. [Crossref] [PubMed]
- Niu DS, Yang J, Xia QF, et al. Thyroid-like low-grade papillary adenocarcinoma of the nasopharynx: A clinicopathological analysis. Modern Oncology 2019;27:3125-7.
- Fu CH, Chang KP, Ueng SH, et al. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx 2008;35:579-82. [Crossref] [PubMed]
- Sillings CN, Weathers DR, Delgaudio JM. Thyroid-like papillary adenocarcinoma of the nasopharynx: a case report in a 19-year-old male. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:e25-8. [Crossref] [PubMed]
- Appukutty SJ, Palma SD, Pitkin L, et al. Thyroid-like low grade nasopharyngeal papillary adenocarcinoma presenting as snoring in a 49-year-old male. Diagnostic Histopathology 2013;19:350-3. [Crossref]
- Ozturk K, Midilli R, Veral A, et al. Primary thyroid-like papillary adenocarcinoma of the nasal septum: a case report. Ear Nose Throat J 2015;94:E19-21. [Crossref] [PubMed]
- Petersson F, Pang B, Loke D, et al. Biphasic low-grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component: report of a case localized to the posterior nasal septum. Head Neck Pathol 2011;5:306-13. [Crossref] [PubMed]
- Liu J, Xu C, Wei J, et al. Thyroid-like Low-grade Nasopharyngeal Papillary Adenocarcinoma: A Case Report. Journal of Chinese Oncology 2019;25:174-6.
- Yao X, Chen X, Zhang R, et al. Clinicopathological analysis of thyroid-like low-grade nasopharyngeal papillary adenocarcinoma. Zhejiang Medical Journal 2018;40:1949-51.
- Zhang D, Wei J, Wang Q. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report and literature review. J Clin Exp Pathol 2017;33:1028-30. [PubMed]
- Dai ZZ, Chen F, Cao C, et al. Clinicopathological analysis of primary thyroid-like low-grade papillary adenocarcinoma of the nasopharynx. Clinical Education of General Practice 2017;15:320-2.
- Liu YF, Huang X, Dan HJ, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: two cases report and literature review. J Clin Exp Pathol 2019;35:216-8.
- Tang YZ, Yu XD, Tang YC, et al. Clinicopathologic features of thyroid-like low-grade nasopharyngeal papillary adenocarcinoma. J Diag pathol 2017;24:503-6.
- García-Gómez J, Sánchez-González F, Pérez-Holgado V, et al. Low-grade papillary adenocarcinoma of nasopharynx. Case report and review of the literature. Acta Otorrinolaringol Esp 2019;70:175-7. [Crossref] [PubMed]
- Chen BN, Wey SL. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma originating from the nasal septum. Int J Clin Exp Med 2018;11:11326-9.



