
Preliminary results on anal cancer by applying intensity modulated radiotherapy and synchronous capecitabine chemotherapy simultaneously
Introduction
Anal cancer is a rare clinical disease with the incidence rate of 1–2/10 million, while it is about 3–4 times that of perianal cancer (1). Surgery has been the standard treatment for anal cancer in the past, that means performing complete resection on patients’ perineum and anus, making an abdominal wall of a permanent fistula. In 1974, Nigro et al. (2) first reported concurrent chemoradiotherapy for the treatment of anal cancer, it’s become the standard treatment for anal cancer (3). Furthermore, previous studies, such as UKCCCR, RTOG8704/ECOG1289, have established that 5 fluorouracil (5-FU)/mitomycin (MMC) concurrent chemoradiotherapy is the standard treatment for anal cancer (4-6). However, concurrent chemoradiotherapy is like a double-edged sword, it improves the curative effect on one hand, yet on the other hand, it also inevitably brings more serious adverse reactions including myelosuppression, thrombocytopenia, radioactive dermatitis, and adverse reaction of the digestive tract and urinary system (4).
In the past, most prospectively randomized studies on radiation therapy of anal carcinoma used 3-D conformal radiotherapy (3DCRT) (4-6). In recent years, intensity modulated radiotherapy (IMRT) has become the mainstream treatment of radiation therapy in various tumors. The IMRT technique is characterized by a highly conformal dose distribution to targets, whereas a constraint dose to organs at risk (OARs) (7). Simultaneous integrated boost IMRT (SIB-IMRT) is one of the techniques of IMRT. It delivered different doses to the gross tumor and regional lymph nodes in a single fraction, reducing the doses to OARs surrounding targets (8). RTOG 0529 was a phase II study to investigate the utility of SIB-IMRT in anal cancer. And the results showed that the 2-year loco-regional control rate was 80% (9). Furthermore, Sakanaka and colleagues analyzed data from ten patients with anal cancer who had been treated with SIB-IMRT and 5-FU/MMC. They found that SIB-IMRT reduced the toxicity of chemoradiotherapy and achieved a high locoregional control (10).
Capecitabine is an oral pro-drug preferentially converted to 5-FU at the tumor site (11). In anal cancer, a number of studies have been published using capecitabine in place of 5-FU (12,13). In the study conducted by Pumpalova et al., overall survival (OS) and cancer-specific survival were equivalent between capecitabine and 5-FU in anal cancer, but patients treated with capecitabine had statistically significant lower incidence of loco-regional relapses (13). In 2013, a phase I study assessed the feasibility and efficacy of SIB-IMRT with concomitant capecitabine and MMC in locally advanced anal cancer (14). However, few studies have focused on the safety and feasibility in anal cancer treated with SIB-IMRT and capecitabine alone.
The purpose of this study was to assess the feasibility, safety and short-term outcome of SIB-IMRT schedule with oral capecitabine in patients with anal cancer. The data about 10 cases were presented in this paper. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2843).
Methods
General clinical data
This is a retrospective observational study. From September 2009 to February 2014, a total of 10 patients with anal cancer received IMRT plus the amount of oral capecitabine chemotherapy during the same period. Inclusion criteria: anal cancer is confirmed by fibre colonoscopy and pathology, and it is also diagnosed by the definition of American Joint Commission on Cancer (AJCC) and the International Union against cancer (UICC); KPS scores should range from 70 to 100; no distant metastasis of the abdominal cavity confirmed by imaging; no previous history of radiotherapy chemotherapy and abdominal cavity. This study was conducted in accordance with the declaration of Helsinki (as revised in 2013) and approved by Ethics Committee of the Seventh Medical Center of PLA General Hospital (No.: 2020-033), and informed consent was taken from all the patients.
Treatment method
Computed tomography (CT) simulation location
The patients fasted and were placed in a supine position and fixed with a thermoplastic membrane. 600 mL of 2% diatrizoate meglumine was taken orally for filling the bladder 90 and 40 min before CT scan, respectively. 100 mL of iodophor was injected intravenously for enhanced scan. The scanning range is from the lower edge of the second lumbar vertebra to the upper 1/3 of the femur, and the thickness of the scanning layer is 5 mm. The images were transferred to ADAC Pinnacle 7.4 treatment planning system.
Target delineation and treatment planning
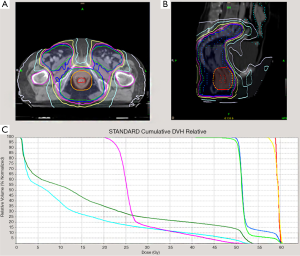
Target delineation was with reference to no. 50 and no. 60 International Commission Radiological Units (ICRU) Report; the gross target volume (GTV) was confirmed by localization of CT image combined with magnetic resonance imaging (MRI), colonoscopy, endorectal ultrasonography and rectal examination, and planning target volume (PTV) 1 is uniformly expanding 20 mm of GTV. While clinical target volume (CTV) is defined as the lymph drainage area of the internal and external iliac as well as the skin of the presacral region, the ischiorectal fossa anal canal and perianal, PTV2 is uniformly expanding 10 mm of CTV. The prescription dose lines should wrap at least 95% PTV; ≥110% dose volume <2%; ≤93% dose volume <3%. PTV1 was given 57.6 Gy per 32 fractions, 1.8 Gy per fraction, once per day; PTV2 was given 49.5 Gy per 32 fractions, 1.55 Gy per fraction, once per day (Figure 1). After 5 weeks of radiotherapy, all patients underwent simulation of secondary CT, there were 2 patients of GTV with gross residual/suspicious residual after radiotherapy, the treatment was finalised by administering a dosage of 1.8 Gy twice (total GTV 61.2 Gy) after a round of initial radiotherapy (Figure 1).
Chemotherapy
Take oral capecitabine from the first day of radiotherapy, 625 mg/m2 per time, twice per day, 5 days per week. No oral capecitabine or other chemotherapy was given after the end of radiotherapy.
Evaluation of side effects and adjustment plan of chemoradiotherapy
Acute and late side effects are graded according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 classification standard. And all patients should have weekly blood routine examination, blood biochemistry and liver function tests during chemoradiotherapy. If the symptoms appear to be grade 3 and 4 of haematologic adverse reactions during chemoradiotherapy, then the chemoradiotherapy should be suspended until it has recovered to grade 1 or 2, and in this case chemoradiotherapy can continue but patients should only take half the dose of capecitabine. If the symptoms appear to be grade 4 of non-haematologic adverse reactions (other than diarrhea), then patients should discontinue capecitabine; if the symptoms appear to be grade 4 of diarrhea, then the chemoradiotherapy should be suspended until it has recovered to grade 1 or 2 of diarrhea, and in this case only radiotherapy can continue.
Endpoints of the study and statistical methods
The primary endpoint was acute adverse reactions and completion of chemoradiotherapy, the secondary endpoint was the recent curative effect of chemoradiotherapy. Survival rates were analyzed with Kaplan-Meier method. Data were analyzed by SPSS21.
Results
Completion of chemoradiotherapy and the acute adverse effects
Six male patients, and 4 female patients were enrolled in this study. All of them aged 34–78 years, and the median age was 53 years. Nine of them suffered from anal squamous carcinoma, and the rest of them suffered from anal canal adenocarcinoma. The related parameters of the patients are shown in the Table 1.
Table 1
| Characters | n (%) |
|---|---|
| Age | |
| Mean age (years) | 53 |
| Range | 34–78 |
| Gender | |
| Male | 6 (60%) |
| Female | 4 (40%) |
| Tumor stage | |
| T1 | 9 (12%) |
| T2 | 5 (48%) |
| T3 | 4 (32%) |
| T4 | |
| Nodal stage | |
| N0 | 5 (50%) |
| N1 | 3 (30%) |
| N2 | 2 (20%) |
| N3 | 0 |
| Distant metastases | |
| M0 | 10 (100%) |
| M1 | 0 |
All of the 10 patients successfully completed the chemoradiotherapy, including 2 cases of secondary CT simulation, there were 2 cases of GTV with gross residual/suspicious residual after radiotherapy, the treatment was finally ended by the administering a dosage of 1.8 Gy × twice after a course of initial radiotherapy. Results showed that there is a rate of 60% (6/10) on grade 1–2 adverse reaction in the blood system, of which there were 5 cases of grade 1 leukopenia or blood platelet decrease, 1 case of grade 2 of white blood cells or platelets decrease. And meanwhile, there’s a rate of 20% (2/10) of incidence of gastrointestinal adverse reactions, which can be defined as grade 1 diarrhea. What’s more, there were no grade 3 or 4 acute adverse reactions which occurred in the blood system and gastrointestinal tract. However, the rate of skin adverse reaction of anus, perineal region and inguinal skin was 100% (10/10), 50% (5/10) were at I–II degree of adverse reactions, while the rest 50% (5/10) were at III degree of adverse reactions. no more than grade 4 toxicity was found in the experience (Table 2). No chemotherapy interruption or dose adjustment due to serious adverse reaction.
Table 2
| Variable | n (%) |
|---|---|
| Acute toxicities, N=10 | |
| Diarrhea | |
| Grade 1 | 2 (20.0) |
| Leukopenia | |
| Grade 1 | 5 (50.0) |
| Grade 2 | 1 (10.0) |
| Dermatitis | |
| Grade 1 | 1 (10.0) |
| Grade 2 | 4 (40.0) |
| Grade 3 | 5 (50.0) |
| Late morbidities, N=10 | |
| Chronic diarrhea | |
| Grade 1 | 1 (10.0) |
Disease control
All the patients were followed up (telephone and/or visits to the hospital), and the median follow-up was 20 months (6–60 months), results showed that the 2-year local control rate was 100% (10/10), survival rate without colostomy was 90% (9/10), distant metastasis free survival rate was 90% (9/10) and OS rate was 90% (9/10). One patient of pre-treatment stage T3N2M0, who was diagnosed as adenocarcinoma, showed distant metastasis (lung and liver metastasis) after the treatment within 20 months, and finally died after 29 months. There’s also one patient of pre-treatment stage (PTV1 was given 57.6 Gy; PTV2 was given 49.5 Gy), exhibited anal canal recurrence after 11 months, after carrying out abdominal perineal combined radical and colostomy, and was disease-free during the following 39 months.
Late reaction
One patient experienced intermittent diarrhea in the 14 months after chemoradiotherapy. No late complications in level of 3 or above were observed in the experiment.
Discussion
Since Nigro et al. (2) reported the chemoradiotherapy for the treatment of anal cancer, radical chemoradiotherapy has become the standard treatment for anal cancer. And what’s more, it is superior to radiotherapy alone (3). RTOG8704/ECOG1289 etc. (4-6) have established that 5-FU/MMC concurrent chemoradiotherapy is the standard treatment for anal cancer.
Capecitabine is an orally administered fluoropyrimidine. It is absorbed in the gastrointestinal tract, which can simulate the effect of continuous infusion of 5-Fu, avoiding side effects and complications of the central venous infusion of 5-Fu (15). Capecitabine can also convert thymidine phosphorylase (TP) into 5-Fu activity in cells. The concentration of TP in tumor cells is higher especially in intestinal tumors, demonstrating that capecitabine has more advantages in the treatment of intestinal tumors (16). Throughout the course of radiotherapy, capecitabine is usually divided into 2 doses, twice a day, 625–825 mg per time. Compared with 5-FU/leucovorin, capecitabine has less acute toxicity. And it’s convenient for oral application. Hence, in the study, we used oral capecitabine for chemotherapy instead of 5-FU/MMC.
The main focus of research in recent years has been to achieve a better local control rate as well as reduce the treatment related side effects. The IMRT technique is characterized by a highly conformal dose distribution to targets, whereas a constraint dose to OARs. So the use of IMRT in the treatment of anal cancer is reasonable and attractive. Recent studies showed that, when compared with the traditional irradiation and three-dimensional conformal radiotherapy, IMRT irradiation can reduce the toxicity related to radiotherapy on the condition of not reducing the effectiveness of anal cancer treatment (1,17,18).
Dose of radiotherapy is an important factor influencing the efficacy of treatment. Improvement in the total dose of radiotherapy is due to random and non-randomly achieved results: control rate of radiotherapy is more useful to smaller tumors than bigger tumors, and by analysis of non-randomized study data, there’s relevance to the dose and control rate, and by retrospective analyzing the data of 50 patients, when the total dose ≥54 Gy, 5-year local control rate and survival rate will be improved (19). However, high doses can lead to serious complications such as severe adverse reaction of the gastrointestinal and urinary tracts, lower extremity oedema, ulcer, fecal incontinence, anal stenosis and perianal necrosis and so on. IMRT can give the target dose of different amounts in the same course of treatment, in theory, on the premise of not prolonging treatment time, it can not only synchronously give a high dose of tumor, but also realize the prophylactic irradiation. In recent years, there were several studies encouraging SIB-IMRT to treat anal cancer, with chemotherapy based on 5-Fu/MMC and the SIB dose of 1.28–2.25 Gy per fraction and total dose of 30.6–59.4 Gy (14,17,18,20).
Lower dose of oral capecitabine (625 mg/m2, twice/day, 5 days/week) and IMRT (PTV1: 1.8 Gy/fraction; PTV2: 1.55 Gy/fraction) were used in this study, total dose was 57.6 Gy/32 fractions. The purpose of this study was to explore a local control rate and ensure the safe and convenient treatment of anal cancer with chemotherapy. From the preliminary results, haematological and gastrointestinal system on the scheme has less adverse reactions, skin and mucous membrane reactions can be accepted, and what’s more, it is encouraging to know that the results of 2-year local control rate, survival rate without colostomy, distant metastasis free survival and OS rate has improved. However, because the number of cases in the group is not enough, together with the fact the observation period is short, the conclusion and the prospect is still to be confirmed by more patients and long-term follow-up.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2843
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2843
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2843). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the declaration of Helsinki (as revised in 2013) and approved by Ethics Committee of the Seventh Medical Center of PLA General Hospital (No.: 2020-033), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vaios EJ, Wo JY. Proton beam radiotherapy for anal and rectal cancers. J Gastrointest Oncol 2020;11:176-86. [Crossref] [PubMed]
- Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6. [Crossref] [PubMed]
- Albuquerque A, Rios E, Schmitt F. Recommendations Favoring Anal Cytology as a Method for Anal Cancer Screening: A Systematic Review. Cancers (Basel) 2019;11:1942. [Crossref] [PubMed]
- Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39. [Crossref] [PubMed]
- Ishida Y, Sakanaka K, Fujii K, et al. Intensity-modulated radiotherapy for cervical esophageal squamous cell carcinoma without hypopharyngeal invasion: dose distribution and clinical outcome. J Radiat Res 2019;60:517-26. [Crossref] [PubMed]
- Franco P, De Bari B, Arcadipane F, et al. Comparing simultaneous integrated boost vs sequential boost in anal cancer patients: results of a retrospective observational study. Radiat Oncol 2018;13:172. [Crossref] [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [Crossref] [PubMed]
- Sakanaka K, Itasaka S, Ishida Y, et al. Dosimetric advantages and clinical outcomes of simultaneous integrated boost intensity-modulated radiotherapy for anal squamous cell carcinoma. Radiat Oncol J 2017;35:368-79. [Crossref] [PubMed]
- Xue W, Wang S, Zhao Z, et al. Short-term outcomes of laparoscopic intersphincteric resection with intraoperative radiotherapy using low-energy X-rays for primary locally advanced low rectal cancer: a single center experience. World J Surg Oncol 2020;18:26. [Crossref] [PubMed]
- Goodman KA, Julie D, Cercek A, et al. Capecitabine With Mitomycin Reduces Acute Hematologic Toxicity and Treatment Delays in Patients Undergoing Definitive Chemoradiation Using Intensity Modulated Radiation Therapy for Anal Cancer. Int J Radiat Oncol Biol Phys 2017;98:1087-95. [Crossref] [PubMed]
- Pumpalova Y, Kozak MM, von Eyben R, et al. Comparison of definitive chemoradiation with 5-fluorouracil versus capecitabine in anal cancer. J Gastrointest Oncol 2019;10:605-615. [Crossref] [PubMed]
- Deenen MJ, Dewit L, Boot H, et al. Simultaneous integrated boost-intensity modulated radiation therapy with concomitant capecitabine and mitomycin C for locally advanced anal carcinoma: a phase 1 study. Int J Radiat Oncol Biol Phys 2013;85:e201-7. [Crossref] [PubMed]
- Kim RD, McDonough S, El-Khoueiry AB, et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur J Cancer 2020;130:219-27. [Crossref] [PubMed]
- Sakai S, Kobuchi S, Ito Y, et al. Assessment of pharmacokinetic variations of capecitabine after multiple administration in rats: a physiologically based pharmacokinetic model. Cancer Chemother Pharmacol 2020;85:869-80. [Crossref] [PubMed]
- Gilbert A, Drinkwater K, McParland L, et al. UK national cohort of anal cancer treated with intensity-modulated radiotherapy: One-year oncological and patient-reported outcomes. Eur J Cancer 2020;128:7-16. [Crossref] [PubMed]
- Dell’Acqua V, Surgo A, Arculeo S, et al. Intensity-modulated radiotherapy (IMRT) in the treatment of squamous cell anal canal cancer: acute and early-late toxicity, outcome, and efficacy. Int J Colorectal Dis 2020;35:685-94. [Crossref] [PubMed]
- Constantinou EC, Daly W, Fung CY, et al. Time-dose considerations with treatment of anal cancer. Int J Radiat Oncol Biol Phys 1997;39:651-7. [Crossref] [PubMed]
- Janssen S, Glanzmann C, Bauerfeind P, et al. Clinical experience of SIB-IMRT in anal cancer and selective literature review. Radiat Oncol 2014;9:199. [Crossref] [PubMed]


