Primary synovial sarcoma of the duodenal bulb: a case report and review of the literature
Introduction
Synovial sarcoma is a type of mesenchymal tumor that originates from soft tissue rather than from synovial tissue. This sarcoma originates from soft tissue, has a special predilection for local recurrence and distant metastasis, and it accounts for 5–10% of all soft tissue tumors (1,2). synovial sarcoma usually occurs in young adults around major joints or tendon sheaths (1) and occasionally in the head and neck (3), lungs (4), heart (5), retroperitoneum (6), prostate (7), within nerves (8), and the kidney (9). Here, we report a 49-year-old Chinese male with primary synovial sarcoma of the duodenal bulb. We also retrieved relevant data on SS in other parts of the digestive system and reviewed the literature. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1107).
Case presentation
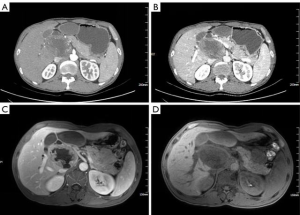
A 49-year-old male presented to our hospital with a 2-month history of upper abdominal pain along with a 4-day amply jaundice. The patient was previously healthy, normotensive, did not have diabetes, and did not have a history of alcohol intake or smoking. According to the blood test results, he had a total bilirubin level of 43.3 µmol/L and a direct bilirubin level of 40.4 µmol/L), a γ-GGT level of 1,798 U/L, an ALP level of 1,454 U/L, and a carbohydrate antigen 19-9 level of 29.16 U/L. A computed tomography (CT) scan of his abdomen revealed that the lump occupied the duodenal bulb wall, compressed the surrounding tissues, and measured roughly 5.0 cm × 7.7 cm × 8.7 cm. The arterial phase of an enhanced CT scan showed that the mass was obviously strengthened and that the branch of the pancreaticoduodenal artery was supplied with sufficient blood (Figure 1A,B). A Magnetic Resonance Imaging (MRI) scan of his abdomen showed that the duodenum bulb contained a large lump with an approximate size of 9.1 cm × 5.5 cm × 7.1 cm; the T1 signal was slightly longer, and the T2 signal was dominant. The arterial phase of an enhanced MRI showed that the mass was obviously strengthened and had an irregular shape (Figure 1C,D). On gastroduodenoscopy, the duodenal bulb and descending portion exhibited no abnormalities. Since the tumor was located between the duodenal walls, it could not be examined by endoscopic ultrasound and needle biopsy. We thus considered a diagnosis of duodenal wall stromal tumor.
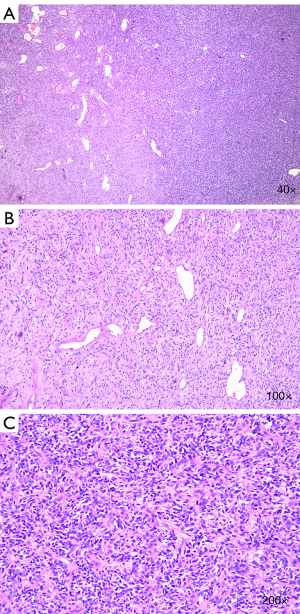
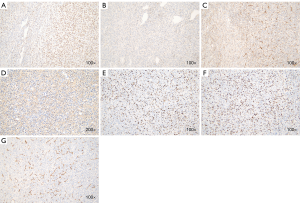
After a multidisciplinary discussion, the patient was originally diagnosed with a malignant duodenal tumor with obstructive jaundice, and consequently, underwent radical pancreatoduodenectomy. Surgical specimens showed that the duodenal papilla had some edema, and a nodular mass observed below the nipple measured 12 cm × 8 cm × 5 cm. The tumor surface was grayish red and gray, and some areas appeared as translucent nodules. The postoperative pathological examination showed that the tumor tissue was fusiform, arranged in a bundle with oval nuclei that were long and fusiform, and contained pink cytoplasm (Figure 2A-C). Immunohistochemistry showed that the tumor was positive for the expression of transducin-like enhancer of split 1 (TLE-1), B-cell lymphoma 2 (Bcl-2), Vimentin, and smooth muscle actin (Figure 3A-D), but was negative for CD34 (Figure 3E), Desmin and S-100 protein; 67% of cells were Ki-67-positive (Figure 3F,G). These pathological findings were indicative of the diagnosis of synovial sarcoma, but still did not provide sufficient diagnostic evidence.
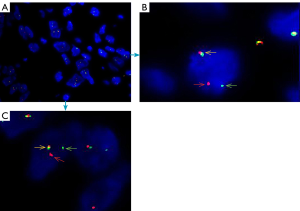
Cytogenetic and fluorescence in situ hybridization (FISH) examination were performed on formalin-fixed paraffin-embedded tumor specimens. FISH was used to detect the fusion of the SSX gene, which is located on the X chromosome, and the SS18 gene (SYT) located on chromosome 18; this fusion produces the SS18 (SYT)-SSX fusion gene (Figure 4A,B,C) and thus, we detected SS18 gene translocations (Figure 4A,B,C). Therefore, FISH demonstrated the t(X;18) (SYT-SSX) gene rearrangement and confirmed the above diagnosis. No such lesions were found on preoperative examination, so a diagnosis of primary duodenal synovial sarcoma was made. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
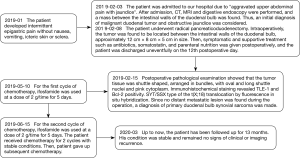
At the time of the reported case, the patient had been treated with ifosfamide twice in the 3 months after surgery. The dose of ifosfamide was 2 g/time for 5 days, and the interval between two chemotherapy was 1 month. After 13 months of follow-up, his condition was stable and remained no signs of clinical or imaging recurrence, and timely follow-up has been conducted. The patient’s treatment and follow-up timeline was shown in Figure 5.
Discussion
Synovial sarcoma is a type of mesenchymal tumor that originates in soft tissue rather than from synovial tissue. synovial sarcoma is divided into three tissue types (2). One is the monophasic type, which consists only of spindle-shaped blastomas arranged in bundles, vortices, and herringbone. Another is the biphasic type, which is composed of different proportions of epithelial cells and spindle cells. The other is the poorly differentiated type, which is characterized by darkly stained oval or round cells, as seen in other small round-cell tumors. synovial sarcoma is difficult to diagnose and is easily misdiagnosed, which is closely related to the diagnostic methods used. CT, MRI, and digestive endoscopy are used to diagnose SS of the digestive system, but considering the current state of the technology, it is difficult to make a definitive diagnosis before surgery. Since the diagnosis of synovial sarcoma relies on histology, immunohistochemistry [TLE-1 and Bcl-2 positivity can indicate a diagnosis of synovial sarcoma (10,11)], and cytogenetics, in most cases, the disease is confirmed using the above three methods to examine surgical specimens. Moreover, 90% of SS is associated with the t(X;18)(p11.2;q11.2) chromosomal translocation, which results in the fusion of the SYT gene on chromosome 18 with the SSX1 or SSX2 gene on the X chromosome. This results in the SYT-SSX fusion protein under certain conditions (cytogenetics, fluorescence in situ hybridization, and RT-PCR can detect this fusion). If the translocation can be identified in tumor tissue, then histology can be used to make a diagnosis (12). The molecular test results of SYT-SSX were positive in our case (Figure 4A,B,C), and combined histological and immunohistochemical analyses showed multiple characteristics of monophasic synovial sarcoma. Therefore, the diagnosis of synovial sarcoma of the duodenal bulb was established (2,10,11). The differential diagnoses of synovial sarcoma include digestive lymphoma, gastrointestinal stromal tumor (GIST), giant juvenile polyp, sarcoma, and ectopic pancreas. It is worth mentioning that GIST is the most common mesenchymal cell tumor. Sometimes, it is difficult to identify GIST and gastrointestinal synovial sarcoma under the microscope. The diagnosis thus needs to be confirmed by TLE-1 and Bcl-2 positivity by immunohistochemistry or the presence of the t(X;18) chromosomal translocation by cytogenetics testing.
In this case, confirmation of the diagnosis was not easy. The tumor originated between the intestinal walls of the duodenal bulb, so gastroduodenoscopy failed to detect it, and the location of the tumor was ultimately confirmed by enhanced CT and MRI. Therefore, imaging physician made the diagnosis of duodenal stromal tumor before surgery. After pathological and immunohistochemical examination of the postoperative specimen, the pathologist considered synovial sarcoma, but still could not make a definite diagnosis. FISH examination was not routinely conducted in our institution due to its high cost, however, in order to obtain a definitive diagnosis, we had the patient’s consent and performed FISH on the specimen, which helped us make the final diagnosis of duodenal synovial sarcoma. This case may serve as experience for gastrointestinal surgeons. First, the tumor is located between the intestinal walls of the duodenal bulb, which is rare and made biopsy difficult and we cannot make a correct diagnosis in a timely manner. Second, in the previous reports, the location of duodenal synovial sarcoma was all in the duodenal lumen, but in this case, the lesion occurred between the intestinal walls of the duodenal bulb and could not be found endoscopically, and the same report has not been seen in the past. Therefore, the report of this case helps gastroenterologists to increase vigilance when encountering such diseases to avoid misdiagnosis.
Until now, 33 cases of synovial sarcoma of the digestive system have been reported, including 10 cases in the esophagus, 1 case in the esophagogastric junction, 13 cases in the stomach, 1 case in the gastroduodenal junction, 5 cases in the duodenum, 1 case in the jejunum, 1 case in the ileum, and 1 case in the colon. The four cases previously reported can be found under endoscopy. One case relapsed 8 months after surgery, and three cases lost follow-up results. We first reported the case of synovial sarcoma originating between the intestinal wall of the duodenal bulb, and had a follow-up time of 13 months. The clinical situation of previous cases and our case are summarized in Table 1 (12-33). Symptoms include dysphagia, upper abdominal pain, and bleeding. We can conclude that the median age of patients with synovial sarcoma of the digestive system is 42 years old (range, 14–76 years), and although there is no obvious gender difference, the ratio of males to females is approximately 1:1. The tumor size ranges from 2 to 16 cm. The macroscopic morphology is primarily polypoid. There have been 17 cases of the monophasic type, 12 cases of the biphasic type, and 2 cases of the poorly differentiated subtype. The histological type of 2 cases was not reported in the literature. The monophasic type is common in synovial sarcoma. In the literature, 32 cases were treated with surgery, and 1 report did not describe the treatment. Eight patients received adjuvant chemotherapy after surgery, 3 patients received radiation therapy, and 3 patients received chemotherapy and radiation therapy. The survival of patients in the reported cases ranged from 1 to 224 months.
Table 1
| Author, year, reference | Location | Presenting symptoms | Age, years | Gender | Gross features | Size, cm | Histologic type | Translocation | Treatment | Follow-up status and (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Palmer et al. 1983, (13) | Esophagus | Dysphagia | 75 | Female | Polypoid | 2.5 | Biphasic | – | Surgery + radiotherapy | Died of other cause, 24 |
| Amr et al. 1984, (14) | Esophagus | Dysphagia | 25 | Male | Polypoid | 5 | Biphasic | – | Surgery | Alive without evidence of disease, 36 |
| Bloch et al. 1987, (15) | Esophagus | Dysphagia, dyspnea | 15 | Male | Polypoid | 10 | Biphasic | – | Surgery + radiotherapy | Alive without evidence of disease, 36 |
| Pulpeiro et al. 1988, (16) | Esophagus | – | 24 | Male | – | – | Biphasic | – | Surgery | – |
| Caldwell et al. 1991, (17) | Esophagus | – | 29 | Female | – | – | – | – | Surgery + chemotherapy + radiotherapy | Alive without evidence of disease, 195 |
| Perch et al. 1991, (18) | Esophagus | – | 15 | Male | – | – | Biphasic | – | Surgery + radiotherapy | Alive without evidence of disease, 5 to 6years after surgery |
| Antón-Pacheco et al. 1996, (19) | Esophagus | Dysphagia, weight loss | 14 | Female | Polypoid | 7 | Biphasic | – | Surgery + chemotherapy + radiotherapy | Alive without evidence of disease, 30 |
| Habu et al. 1998, (20) | Esophagus | Sensation of something stuck in his throat | 20 | Male | Polypoid | 8 | Biphasic | – | Surgery + chemotherapy + radiotherapy | Alive without evidence of disease, 20 |
| Bonavina et al. 1998, (21) | Esophagus | Achalasia | 63 | Female | Polypoid | – | – | – | – | – |
| Billings et al. 2000, (22) | Gastroesophageal junction | Incidental finding for pyloric Stenosis | 47 | Female | Polypoid | 5.2 | Biphasic | t(X;18) | Surgery | Alive without evidence of disease, 21 |
| Billings et al. 2000, (22) | Stomach | Abdominal pain, nausea, vomiting | 55 | Female | Spherical intramural | 16 | Biphasic and poorly differentiated synovial sarcoma | t(X;18) | Surgery | Died of other cause, 6 |
| Chan et al. 2004, (23) | Jejunum | Epigastric pain, vomit, fever | 28 | Male | Polypoid intramural | 15 | Monophasic | t(X;18) | Surgery | Died of other cause, 1 |
| Butori et al. 2006, (24) | Esophagus | Dysphagia | 72 | Female | Polypoid | 11 | Biphasic | t(X;18) | Surgery + chemotherapy | 6 |
| Akhunji et al. 2007, (25) | Stomach | Epigastric pain | 42 | Male | – | 11 | Biphasic | t(X;18) | Surgery + chemotherapy | Died of other cause, 24 |
| Parfitt et al. 2007, (26) | Colon | Rectal bleeding | 32 | Male | Polypoid | 2 | Monophasic | t(X;18) | Surgery | 5 |
| Schreiber-Facklam et al. 2007, (27) | Duodenum | Abdominal pain | 39 | Female | Polypoid | 5 | Monophasic | t(X;18) | Surgery + chemotherapy | Recurrence 8 months after surgery |
| Makhlouf et al. 2008, (28) | Stomach | – | 67 | Female | – | 0.8 | Monophasic | t(X;18) | Surgery | Alive without evidence of disease, 12 |
| Makhlouf et al. 2008, (28) | Stomach | – | 49 | Male | – | 2 | Monophasic with a poorly differentiated component | t(X;18) | Surgery | Died of other cause, omental metastasis, 29 |
| Makhlouf et al. 2008, (28) | Stomach | – | 68 | Female | – | 2 | Monophasic | t(X;18) | Surgery | Alive without evidence of disease, 22 |
| Makhlouf et al. 2008, (28) | Stomach | – | 29 | Male | – | 2.8 | Monophasic | t(X;18) | Surgery | Alive without evidence of disease, 224 |
| Makhlouf et al. 2008, (28) | Stomach, gastroduodenal junction | – | 54 | Female | – | 3 | Monophasic | t(X;18) | Surgery | Recent case |
| Makhlouf et al. 2008, (28) | Stomach | – | 58 | Female | – | 3 | Monophasic | t(X;18) | Surgery | Alive without evidence of disease, 21 |
| Makhlouf et al. 2008, (28) | Stomach | – | 37 | Female | – | 4 | Monophasic | t(X;18) | Surgery | Local recurrence, re-excised. died of other cause, 48 |
| Makhlouf et al. 2008 (28) | Stomach | – | 50 | Male | – | 6 | Monophasic | t(X;18) | Surgery + chemotherapy | Alive with recurrence, 6 |
| Makhlouf et al. 2008, (28) | Stomach | – | 42 | Male | Polypoid | 8 | Biphasic | t(X;18) | Surgery + chemotherapy | Died of other cause, 25 |
| Makhlouf et al. 2008, (28) | Stomach | – | 66 | Female | Polypoid | 15 | Monophasic | t(X;18) | Surgery | Lost to follow up |
| Borens-Fefer et al. 2009, (29) | Duodenum | Pain | 14 | Male | – | 5 | Monophasic | t(X;18) | Surgery + chemotherapy | Lost to follow up |
| Company Campins et al. 2009, (12) | Proximal duodenum | Weight loss Asthenia anorexia, nausea, epigastric pain | 69 | Female | Spherical intramural | 8 | Monophasic | t(X;18) | Surgery | Died due to complications, 1 |
| García Ruiz et al. 2010 (30) | Duodenal | Pain | 70 | Male | Polypoid | 9 | Biphasic | t(X;18) | Surgery | Lost to follow up |
| Alsharief et al. 2012, (31) | Ileum | Abdominal pain, distension and heaviness | 29 | Female | Intramural | 8 | Monophasic | – | Surgery | Alive without evidence of disease, 6 |
| Wang et al. 2012, (32) | Stomach | Abdominal pain | 38 | Female | – | 7 | Monophasic | t(X;18) | Surgery + chemotherapy | Alive without evidence of disease |
| Sahara et al. 2013, (33) | Stomach | Abdominal pain | 22 | Male | – | – | Monophasic | t(X;18) | Surgery | Lost to follow up |
| Present case 2019 | Duodenal bulb | Abdominal pain | 49 | Male | – | 12 | Monophasic | t(X;18) | Surgery + chemotherapy | Alive without evidence of disease, 13 (continue to follow) |
Surgery is still the primary treatment for synovial sarcoma of the digestive system without tumor metastasis. Chemotherapy and radiation therapy are mainly used for preoperative and postoperative adjuvant therapy. Ifosfamide and doxorubicin are the first-line chemotherapeutic drugs, while pazopanib is a second-line drug. Although pazopanib is the only tyrosine kinase inhibitor approved by the FDA for the treatment of synovial sarcoma, due to the resistance of tumors to pazopanib, its therapeutic effect is not satisfactory. In recent studies, the over-activation of IGF-1 and insulin receptor (IGF1R/InsR) was shown to alter the AKT and ERK pathways in synovial sarcoma cells. AKT and ERK activity was reduced, which in turn reduced the resistance of tumor cells to pazopanib (34). In recent years, radiation therapy has rarely been used in the treatment of synovial sarcoma of the digestive system. Research on targeted therapy has received substantial attention, and recent studies by Isfort et al. (35) confirmed that activation of YAP/TAZ signaling is a common pattern in SS and is functionally dependent on the SS18-SSX fusion protein, which interferes with YAP. The interference in signal transduction of TAZ through small molecule inhibitors may provide a new and effective way for the treatment of synovial sarcoma. synovial sarcoma also expresses survivin. The BirM 5 promoter induced by YM 155 can change its activity, and thus, survivin may be a feasible target for the treatment of synovial sarcoma (36). In summary, the above research mechanism requires further clarification, and its clinical application is still in the experimental stage. Whether it can be used for the treatment of digestive tract synovial sarcoma has not been confirmed by relevant literature or clinical data. We should accelerate clinical big data studies, make breakthroughs on the basis of existing research, and strive to use the above research results for the treatment of synovial sarcoma in the digestive tract and elsewhere. At present, there are few reports on duodenal synovial sarcoma, and there are no relevant guidelines for postoperative chemotherapy. We used ifosfamide for chemotherapy in this patient according to the chemotherapy regimen for synovial sarcoma of other tissues. The patient was treated with chemotherapy twice without significant adverse reactions. Up to now, there was no recurrence or metastasis.
The 5- and 10-year survival rates of patients with synovial sarcoma of bone tissue are 76.4% and 60.4%, respectively, which are related to tumor size, tumor grade, chemotherapy, and radiation therapy (37). Since synovial sarcoma of the digestive system is difficult to diagnose, easily misdiagnosed, has a high degree of malignancy, and few cases are seen, there is no relevant literature that reports the long-term survival rate of patients with this disease. After following up for 13 months, our case was no recurrence or metastasis. Bergh et al. (38) divided synovial sarcoma patients into a low-risk group (patients age <25 years, tumor size <5 cm, histological classification as monophasic or biphasic) and a high-risk group (patient age <25 years, tumor size >5 cm, histological classification as poorly differentiated). It has also been suggested that the genotyping and prognostic correlation of SS remains uncertain (39). synovial sarcoma appears in the head and neck, trunk, lung pleura, and peritoneal organs, and areas other than the limbs usually have a worse prognosis. Moreover, tumor size (>5 cm), nerve infiltration, vascular invasion, P53 overexpression, and high expression of Ki-67 increase the risk of SS metastasis and recurrence (40-42). We would like to know if the prognosis of synovial sarcoma of the digestive system is related to the above-mentioned factors. Unfortunately, no clinical data have demonstrated the prognostic factors of digestive system synovial sarcoma. Therefore, in order to solve this problem, we should collect more cases and join more medical institutions to make a breakthrough in the prognosis of synovial sarcoma in digestive system.
In conclusion, primary synovial sarcoma of the digestive system is rare and easily misdiagnosed. We reported the first case of synovial sarcoma arising between the intestinal walls of the duodenal bulb with a concomitant SYT/SSX type of the t(X;18) translocation. At present, there are few reports on the diagnosis and treatment of duodenal synovial sarcoma. We hope to deepen our general perception and understanding of synovial sarcoma through report of this rare sarcoma.
Acknowledgments
We would like to thank all members of the Department of Pathology, Lanzhou University Second Hospital, Lanzhou, Gansu, China, for their expert technical assistance.
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1107
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1107). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sultan I, Rodriguez-Galindo C, Saab R, et al. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer 2009;115:3537-47. [Crossref] [PubMed]
- Suurmeijer AJH, de Bruijn D, Geurts van Kessel A, et al. Synovial Sarcoma. Lyon: IARC Press; 2013.
- Park JK, Ham SY, Hwang JC, et al. Synovial sarcoma of the head and neck: a case of predominantly cystic mass. AJNR 2004;25:1103-5. [PubMed]
- Etienne-Mastroianni B, Falchero L, Chalabreysse L, et al. Primary sarcomas of the lung: a clinicopathologic study of 12 cases. Lung Cancer 2002;38:283-9. [Crossref] [PubMed]
- Eswaran P, Devadoss P, Narasimhan LS, et al. Synovial sarcoma of the heart: A case report and literature review. J Cancer Res Ther 2015;11:659. [Crossref] [PubMed]
- Chatzipantelis P, Kafiri G. Retroperitoneal synovial sarcoma: a clinicopathological study of 6 cases. J Buon 2008;13:211-6. [PubMed]
- Jun L, Ke S, Zhaoming W, et al. Primary synovial sarcoma of the prostate: report of 2 cases and literature review. Int J Surg Pathol 2008;16:329-34. [Crossref] [PubMed]
- Chu PG, Benhattar J, Weiss LM, et al. Intraneural synovial sarcoma: two cases. Mod Pathol 2004;17:258-63. [Crossref] [PubMed]
- Tranesh G, Cortese C, Thiel D, et al. Primary synovial sarcoma of the kidney-A case report and literature review. Int J Clin Exp Pathol 2018;11:2241-5. [PubMed]
- Jagdis A, Rubin BP, Tubbs RR, et al. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol 2009;33:1743-51. [Crossref] [PubMed]
- Barrott JJ, Zhu JF, Smith-Fry K, et al. The Influential Role of BCL2 Family Members in Synovial Sarcomagenesis. Mol Cancer Res 2017;15:1733-40. [Crossref] [PubMed]
- Company Campins MM. Primary monophasic synovial sarcoma of the duodenum confirmed by cytogenetic analysis with demonstration of t(X;18): a case report. J Gastrointestin Liver Dis 2009;18:89-93. [PubMed]
- Palmer BV, Levene A, Shaw HJ. Synovial sarcoma of the pharynx and oesophagus. J Laryngol Otol 1983;97:1173-6. [Crossref] [PubMed]
- Amr SS, Shihabi NK, Al Hajj H. Synovial sarcoma of the esophagus. Am J Otolaryngol 1984;5:266-9. [Crossref] [PubMed]
- Bloch MJ, Iozzo RV, Edmunds LH Jr, et al. Polypoid synovial sarcoma of the esophagus. Gastroenterology 1987;92:229-33. [Crossref] [PubMed]
- Pulpeiro JR, Cruz R, Arenas A, et al. Para-oesophageal synovial sarcoma. Eur J Radiol 1988;8:120-1. [PubMed]
- Caldwell CB, Bains MS, Burt M. Unusual malignant neoplasms of the esophagus. Oat cell carcinoma, melanoma, and sarcoma. J Thorac Cardiovasc Surg 1991;101:100-7. [Crossref] [PubMed]
- Perch SJ, Soffen EM, Whittington R, et al. Esophageal sarcomas. J Surg Oncol 1991;48:194-8. [Crossref] [PubMed]
- Antón-Pacheco J, Cano I, Cuadros J, et al. Synovial sarcoma of the esophagus. J Pediatr Surg 1996;31:1703-5. [Crossref] [PubMed]
- Habu S, Okamoto E, Toyosaka A, et al. Synovial sarcoma of the esophagus: report of a case. Surg Today 1998;28:401-4. [Crossref] [PubMed]
- Bonavina L, Fociani P, Asnaghi D, et al. Synovial sarcoma of the esophagus simulating achalasia. Dis Esophagus 1998;11:268-71. [Crossref] [PubMed]
- Billings SD, Meisner LF, Cummings OW, et al. Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol 2000;13:68-76. [Crossref] [PubMed]
- Chan GS, Yuen ST, Chan KW. Synovial sarcoma presenting as a polypoid jejunal mass. Histopathology 2004;44:191-3. [Crossref] [PubMed]
- Butori C, Hofman V, Attias R, et al. Diagnosis of primary esophageal synovial sarcoma by demonstration of t(X;18) translocation: a case report. Virchows Arch 2006;449:262-7. [Crossref] [PubMed]
- Akhunji S, Musil I, Baisre de Leon A, et al. Synovial sarcoma arising in the gastric wall: case report and literature review. Cancer Therapy 2007;5:457-62.
- Parfitt JR, Xu J, Kontozoglou T, et al. Primary monophasic synovial sarcoma of the colon. Histopathology 2007;50:521-3. [Crossref] [PubMed]
- Schreiber-Facklam H, Bode-Lesniewska B, Frigerio S, et al. Primary monophasic synovial sarcoma of the duodenum with SYT/SSX2 type of translocation. Hum Pathol 2007;38:946-9. [Crossref] [PubMed]
- Makhlouf HR, Ahrens W, Agarwal B, et al. Synovial sarcoma of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 10 cases. Am J Surg Pathol 2008;32:275-81. [Crossref] [PubMed]
- Borens-Fefer B, Lingier P, Cassart M, et al. Synovial sarcoma of the duodenum in adolescent. JBR-BTR 2009;92:142-3. [PubMed]
- García Ruiz S, Jiménez Rodríguez RM, Alcaide León P, et al. Sunovial two-phase sarcoma in third portion of the duodenum: clinical case and review of the literature. Rev Esp Enferm Dig 2010;102:62-3. [Crossref] [PubMed]
- Alsharief AN, Fageeh M, Alabdulkarim Y. Monophasic synovial sarcoma presenting as a primary ileal mass: a case report and review of the literature. J Med Case Rep 2012;6:83. [Crossref] [PubMed]
- Wang CC, Wu MC, Lin MT, et al. Primary gastric synovial sarcoma. J Formos Med Assoc 2012;111:516-20. [Crossref] [PubMed]
- Sahara S, Otsuki Y, Egawa Y, et al. Primary synovial sarcoma of the stomach--a case report and review of the literature. Pathol Res Pract 2013;209:745-50. [Crossref] [PubMed]
- Lanzi C, Dal Bo L, Favini E, et al. Overactive IGF1/Insulin Receptors and NRASQ61R Mutation Drive Mechanisms of Resistance to Pazopanib and Define Rational Combination Strategies to Treat Synovial Sarcoma. Cancers 2019;11:408. [Crossref] [PubMed]
- Isfort I, Cyra M, Elges S, et al. SS18-SSX-Dependent YAP/TAZ Signaling in Synovial Sarcoma. Clin Cancer Res 2019;25:3718-31. [Crossref] [PubMed]
- Mika A, Luelling SE, Pavek A, et al. Epigenetic Changes at the Birc5 Promoter Induced by YM155 in Synovial Sarcoma. J Clin Med 2019;8:408. [Crossref] [PubMed]
- Outani H, Nakamura T, Murata H, et al. Localized synovial sarcoma: A single institutional study of 191 patients with a minimum follow-up of 5 years for survivors. J Surg Oncol 2019;119:850-5. [Crossref] [PubMed]
- Bergh P, Meis-Kindblom JM, Gherlinzoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer 1999;85:2596-607. [Crossref] [PubMed]
- Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 2004;22:4040-50. [Crossref] [PubMed]
- Antonescu CR, Leung DH, Dudas M, et al. Alterations of cell cycle regulators in localized synovial sarcoma: A multifactorial study with prognostic implications. Am J Pathol 2000;156:977-83. [Crossref] [PubMed]
- Lewis JJ, Antonescu CR, Leung DH, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol 2000;18:2087-94. [Crossref] [PubMed]
- Trassard M, Le Doussal V, Hacène K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 2001;19:525-34. [Crossref] [PubMed]