
EDIL3 regulates gastric cancer cell migration, invasion and epithelial-mesenchymal transition via TGF-β1/XIST/miR-137 feedback loop
Introduction
Gastric cancer is a serious public health problem all over the world (1). Up to date, the five-year survival rate of patients with gastric carcinoma is low, indicating a poor prognosis of gastric cancer (2,3). It is important that the barriers of effective therapy in gastric cancer are recurrence in cancer early stage and metastasis. Furthermore, epithelial-mesenchymal transition (EMT) is well-known to display key roles in the metastasis and relapse of cancers (4,5). Therefore, in addition to the basic biological characteristics such as proliferation, migration as well as invasion, the inhibition of EMT is another strategy to control the development of the gastric cancer (6,7).
Epidermal growth factor-like repeats and discoidin I-like domain 3 (EDIL3) is an extracellular matrix protein and is identified as a novel regulator of EMT, which contributes to migration and invasion (8,9) and has been reported to be upregulated in various types of human cancers and closely related with tumors progression (9,10). Additionally, EDIL3 is identified as a novel regulatory factor, which exhibits a correlation of elevated EDIL3 expression and poor prognosis in several tumors (9). However, EDIL3 expression in gastric cancer and potential functions on gastric cancer remain to be explored in depth.
It has been reported that EDIL3 participates in the proliferation and EMT of human lens epithelial cells, and the underlying mechanism might be related with regulating the transforming growth factor-β (TGF-β) signaling pathway (11). Also, TGF-β1 could regard as a critical modulator of the X-inactive specific transcript (XIST) (12). Non-coding RNAs including microRNAs (miRNAs) and long-chain non-coding RNAs (lncRNAs) play critical regulatory roles in the development of cancers. Specifically, in hepatic cellular cancer, miR-137 downregulation triggers EMT to promote the migration and invasion of hepatic cellular cancer by upregulating the expression level of EDIL3 (13). Moreover, a previous report revealed that lncRNA XIST was upregulated in gastric carcinoma and possessed the promotion of growth and invasion in cancer cells (14,15). Besides, miR-137 is a potential target of XIST, and their direct binding is basic molecular mechanism underlying the control of the angiogenesis of neuroglioma (16). But the functions of XIST/miR-137 axis on gastric cancer are not fully elaborated.
Therefore, the present investigation was conducted to illustrate whether EDIL3 can modulate human gastric cancer cell proliferation, migration, invasion and EMT by the regulation of TGF-β1/lncRNA XIST/miR-137 feedback loop. In this study, we found that expression of EDIL3 was increased in gastric cancer tissues and cell lines. EDIL3 regulated gastric cancer cell proliferation, migration, invasion and EMT, which was involved in the regulation of TGF-β1/XIST/miR-137 feedback loop, and EDIL3 knockdown inhibited tumor growth in nude mice. Our findings provide a better understanding of the pathways involved in the development of gastric cancer and novel targets for the treatment of gastric cancer.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2967b).
Methods
Tissue specimens
A total of 83 patients with gastric cancer at the Department of General Surgery of Guizhou Provincial People’s Hospital were enrolled in the present study from March 2012 to July 2014. Specimens of tumor tissues and adjacent paracancerous histological normal tissues (PCHNTs) were collected during surgery. The PCHNT specimens were extracted >3 cm from the tumor margin and assessed microscopically for the presence of normal cells and the absence of dysplastic cells. Following surgical removal, the fresh specimens was preserved at −80 °C after freezing in liquid nitrogen for further analysis by western blot or RT-qPCR. Analyzation of the correlation between EDIL3 expression and the clinicopathological characteristics of gastric cancer patients was showed in Table 1. None of the patients with gastric cancer had received preoperative radiotherapy or chemotherapy prior to surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Guizhou Provincial People’s Hospital and informed consent was taken from all the patients. Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Table 1
| Clinical characteristics | Case number | EDIL3 | P value | |
|---|---|---|---|---|
| High | Low | |||
| Gender | 0.289 | |||
| Male | 50 | 31 | 19 | |
| Female | 33 | 16 | 17 | |
| Age | 0.462 | |||
| ≤60 years | 50 | 30 | 20 | |
| >60 years | 33 | 17 | 16 | |
| Lymph node metastasis | 0.0289 | |||
| No | 28 | 13 | 15 | |
| Yes | 55 | 40 | 15 | |
| TNM stage | 0.013 | |||
| I | 21 | 7 | 14 | |
| II | 14 | 6 | 8 | |
| III | 48 | 26 | 22 | |
| Tumor differentiation | 0.0019 | |||
| High and medium | 23 | 9 | 14 | |
| Low and none | 60 | 46 | 14 | |
| Tumor size | 0.1010 | |||
| ≤4 cm | 49 | 28 | 21 | |
| >4 cm | 34 | 26 | 8 | |
Cell culture
Human normal gastric epithelial cells GES-1 and human gastric cancer cells SGC-7901, BGC-823, AGS as well as MKN-45 were obtained from American Type Culture Collection (ATCC). GES-1 and BGC-823 were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (Gibco, USA) and 1% mixture of penicillin and streptomycin (Sigma, USA), while SGC-7901, AGS and MKN-45 were cultured in Roswell Park Memorial Institute (RPMI) 1640 containing 10% fetal bovine serum (Gibco, USA) and 1% mixture of penicillin and streptomycin (Sigma, USA). All the cell lines were incubated in a 5% CO2 incubator, at 37 °C. For TGF-β1 treatment, AGS cells were treated with Decorin, TGF-β1 inhibitor (10µM Sigma, USA) or TGF-β1 (R&D Systems, Minneapolis, MN USA).
RNA extraction and real-time quantitative polymerase chain reaction (RT-qPCR)
The transcription levels of EDIL3, XIST and miR-137 were evaluated by RT-qPCR assay. A commercial RNA extraction kit obtained from TaKaRa Bio Co. Ltd. was used to extract RNA from indicated tissues and cell lines. Next, the total RNA was produced into cDNA using a Prime Script RT Master Mix (Takara, China). Thirdly, The RT-qPCR assay was carried out using a SYBR-Green system (Takara), in agreement with the kit’s instructions. Finally, the 2−ΔΔCt method was performed to obtain the transcriptional levels of EDIL3, XIST and miR-137. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference gene for EDIL3 and XIST, and U6 was used as the internal reference gene for miR-137. The primer sequences of EDIL3, XIST, miR-137, U6 and GAPDH used in this study were showed in Table 2.
Table 2
| Name | Sequence (5'-3') | Length |
|---|---|---|
| XIST | F: GCTCTTCATTGTTCCTATCTGCC | 23 |
| R: TGTGTAAGTAAGTCGATAGGAGT | 23 | |
| GAPDH | F: CCAGGTGGTCTCCTCTGA | 18 |
| R: GCTGTAGCCAAATCGTTGT | 19 | |
| miR-137 | F: GCGCGCTTATTGCTTAAGAATAC | 23 |
| R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTACGC | 50 | |
| EDIL3 | F: ACTGTCGGGTTGTTCTGAGC | 20 |
| R: AGGTCCAGGCATTCACTTTG | 20 | |
| U6 | F: CTCGCTTCGGCAGCACA | 17 |
| R: AACGCTTCACGAATTTGCGT | 20 |
Cell transfection
The short hairpin (sh) RNA-XIST vectors, shRNA-EDIL3 vectors, miR-137 mimics, EDIL3 or XIST overexpression vectors and corresponding negative control vectors were prepared by GenePharma (Shanghai, China). Subsequently, the AGS or MKN-45 cells were transfected with shRNA-EDIL3 vectors, shRNA-XIST vectors, miR-137 mimics, EDIL3 or XIST overexpression vectors using Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer’s instructions.
Western blot
Specimens, AGS or MKN-45 cells were lysed by radioimmunoprecipitation assay (RIPA) lysis buffer (Beotime, China). After determination, the concentrations of protein samples were adjusted to be equal. Then the protein samples were electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transferred to nitrocellulose membranes (Millipore, USA). Next, the 5% skimmed milk was used to incubate the membranes for 1 h, and then they were incubated with the primary antibodies for EDIL3 (ab88667), E-cadherin (ab40772), N-cadherin (ab76011), Vimentin(ab92547), Snail (ab216347) and GAPDH (ab8245) were purchased from Abcam (Cambridge, MA, USA) for overnight at 4 °C. After washed with tris-buffered saline and Tween 20 (TBST) for 3 times, the membranes were then incubated with horseradish-peroxidase (HRP)-conjugated goat anti-rabbit-IgG (sc-2004, Santa Cruz Biotechnology, Inc.) for 2 h. The bands were assessed by a commercial electrochemiluminescence detection regent (Pierce Biotechnology, USA). Protein levels were determined by normalization to GAPDH.
MTT assay
AGS or MKN-45 cells were cultured in 96-well plates. Then the medium was removed and 100 µL fresh medium and 10 µL MTT solution (Beyotime, China) were supplemented into each well. After another 4 h incubation, the medium containing MTT solution was removed and 150 µL dimethyl sulfoxide (DMSO) was added to each cell well. A gentle shake for 10 min was performed to promote the dissolution of crystals. Finally, the absorbance was determined by a spectrophotometer at 490 nm.
Flow cytometric analysis
The cells were harvested and washed in PBS and fixed in ice-cold 70% ethanol for 1 h. Following treatment with RNase A (50 µg/mL; Sigma-Aldrich), Then, the cells were stained with propidium iodide (PI; Sigma-Aldrich) for 30 min at room temperature and then analyzed and recorded using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Cell cycle analysis was performed using FlowJo software (TreeStar Inc., Ashland, OR, USA).
Colony formation assay
AGS or MKN-45 cells were transfected with corresponding vectors and treated with TGF-β1 inhibitor or TGF-β1. After the prior treatment, the RPMI 1640 was replaced with fresh culture medium for a 7-day incubation in the normal condition under. Then the culture medium was discarded and subsequently the formed colonies were fixed. The crystal violet solution was used to stain the cells, followed by the image record for the assessment of positive cell number.
Transwell assay
Both migration and invasion of AGS or MKN-45 cells were examined by transwell assay. AGS or MKN-45 cells were seeded into the top of chambers. Importantly, the chambers coated with Matrigel were used for invasion evaluation and those uncoated with Matrigel were used for migration evaluation. After corresponding treatments, cotton swabs were used to remove AGS or MKN-45 cells on the top ones. Meanwhile, paraformaldehyde was used to fix AGS or MKN-45 cells on the lower of the top chambers, which subsequently stained with 0.5% of crystal violet solution. Next, the number of stained cells as positive cells were measured.
Dual-luciferase reporter assay
Dual-luciferase reporter assay was used to confirm XIST binding to miR-137, and miR-137 binding to EDIL3. The wild-type and mutant 3'UTR segments of EDIL3 or wild-type and mutant segments of XIST were constructed into pmirGLO, then cotransfected into HEK293T cell along with miR-137 mimics or mimics NC using Lipofectamine 2000. The luciferase activity was detected by a dual-luciferase reporter assay system (Promega, USA).
Tumorigenesis in nude mice
BALB/c nude mice (8–12 weeks old, 22±3 g) were purchased from the Experimental Animal Center of Guizhou University (Guizhou, China) and were randomized into two groups (20 mice in each group). AGS cell suspensions (0.25 mL) infected with sh-EDIL3 or sh-NC (5×106 cells/ mouse) were subcutaneously injected into the armpits or the backs of the mice. Tumor sizes were recorded every 3 days for 4 weeks including maximum diameter (A, mm) and vertical short diameter (B, mm). The tumor volumes were calculated using the formula V (mm3) = 1/2(A×B2). After 28 days of observation, the mice were sacrificed, and the xenograft tumors were removed and weighed for analysis. Experiments were performed by the Committee on the Ethics of Animal Experiments of Guizhou Provincial People’s Hospital, in compliance with National Institutes of Health guidelines for the care and use of animals.
Statistical analysis
All experimental data were presented as mean ± standard deviation (SD). The independent experiments were performed at least 3 times. The differences were analyzed by SPSS 21.0. Student’s t-test was used to analyze two independent groups and a one- or two-way analysis of variance (ANOVA) was used to analyze multiple groups. A 5-year survival curve was constructed using the Kaplan-Meier method. Statistical significance was evaluated with the log-rank test. If the P value was less than 0.05, it could be considered as statistically significant.
Results
Knockdown of EDIL3 inhibits proliferation, migration, invasion and EMT in gastric cancer cells
Western blot analysis of EDIL3 expression in 8 representative paired samples of gastric cancer tissues and PCHNTs, and the results suggested that EDIL3 expressed higher in gastric cancer tissues, compared with normal gastric tissues (Figure 1A). The survival statuses of 83 patients with gastric cancer were evaluated by Kaplan-Meier survival curves and survival analysis showed that patients with low EDIL3 expression had a significantly better survival compared to patients with high EDIL3 expression (Figure 1B, log-rank-test P = 0.03). RT-qPCR assay was also used to determine the EDIL3 mRNA level in normal gastric epithelial cells GES-1 and gastric cancer cells SGC-7901, BGC-823, AGS as well as MKN-45. Next, we selected the AGS and MKN-45 cells with the highest expression level of EDIL3 as the further research model (Figure 1C). After building the shRNA expression vectors and transfecting them into AGS and MKN-45 cells for EDIL3 knockdown, the EDIL3 expression level was dramatically decreased by western blot analysis (Figure 1D). MTT assay indicated that the proliferation of AGS and MKN-45 cells was restrained by EDIL3 knockdown (Figure 1E). The downregulation of EDIL3 expression led to gastric cancer cell cycle arrest in the G1 phase (Figure 1F). Moreover, all the colony formation capability, migration and invasion of AGS cells were markedly inhibited after EDIL3 knockdown (Figure 1G,H). Besides, RT-qPCR analysis showed that knockdown of EDIL3 restrained the XIST expression and elevated the expression level of miR-137 (Figure 1I). As shown in Figure 1J, the decreased relative levels of EDIL3, Snail, N-cadherin as well as Vimentin and the raised expression level of E-cadherin were observed after EDIL3 downregulation. Collectively, knockdown of EDIL3 negatively regulates AGS and MKN-45 cell biological characteristics such as proliferation, migration, invasion and EMT. In addition, the expression of EDIL3 in gastric tumors did not correlate with gender, age or the size of the primary gastric tumor. However, the expression of EDIL3 in primary gastric tumors was associated with lymph node metastasis, TNM stage and tumor differentiation (Table 1).
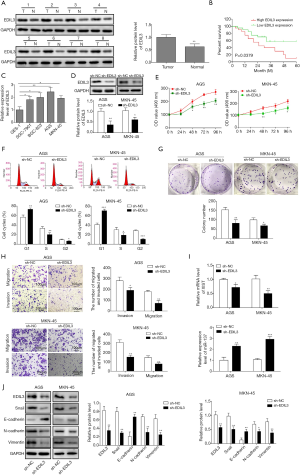
Downregulation of XIST inhibits proliferation, migration, invasion and EMT in gastric cancer cells
The relative level of XIST was significantly reduced after the shRNA-XIST vectors were transfected into AGS and MKN-45 cells (Figure 2A). Subsequently, after XIST was downregulated, the cell growth, colony formation capability, migration and invasion were all suppressed and cell cycle arrest induced (Figure 2B,C,D,E). The western blot analysis suggested an increase in E-cadherin level and decreases in the expression levels of EDIL3, Snail, N-cadherin, and Vimentin triggered by knockdown of XIST (Figure 2F). The data in Figure 2G showed that knockdown of XIST led to inhibition of EDIL3 expression and promotion of miR-137 expression in AGS and MKN-45 cells. Lastly, the dual-luciferase reporter analysis demonstrated that XIST could bind to miR-137 (Figure 2H). Therefore, it revealed that inhibition of XIST expression results in inhibitory effects on proliferation, migration, invasion and EMT in gastric cancer cells and miR-137 is the target of XIST.
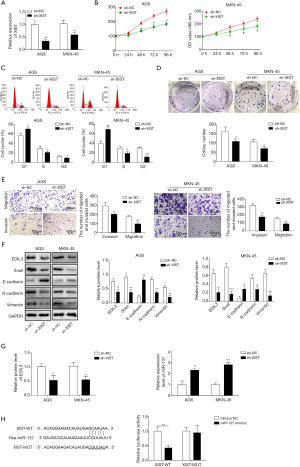
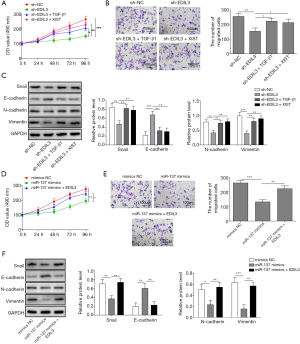
Overexpression of miR-137 exerts inhibitory effects on proliferation, migration, invasion and EMT in gastric cancer cells
After transfection of miR-137 mimics into AGS and MKN-45 cells, the abundance of miR-137 was increased, indicating that the miR-137 overexpression vector was constructed successfully (Figure 3A). The proliferation, colony formation ability, migration and invasion in AGS and MKN-45 cells were evidently suppressed and cell cycle arrest induced by miR-137 overexpression (Figure 3B,C,D,E). Also, the expression levels of EDIL3, Snail, N-cadherin, and Vimentin on protein level were decreased by miR-137 overexpression, but that of E-cadherin was increased through the upregulation of miR-137 (Figure 3F). Furthermore, we found that miR-137 overexpression inhibited the expressions of EDIL3 and XIST (Figure 3G). Importantly, the direct binding of EDIL3 to miR-137 was confirmed (Figure 3H). These findings revealed that miR-137 overexpression induces inhibitory effects on proliferation, migration, invasion and EMT in gastric cancer cells and EDIL3 is the target of miR-137.
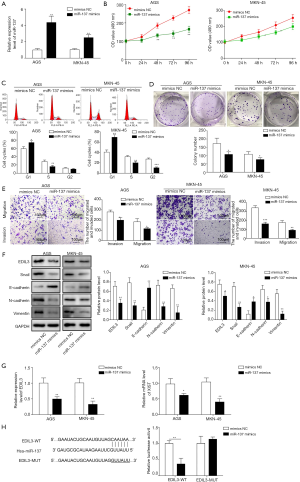
TGF-β1 stimulates XIST expression and inhibits miR-137 expression
TGF-β1 acts as a growth inhibitor in normal cells, whereas it promotes tumor growth and progression in tumor cells. AGS cells were exposed to TGF-β1 at various concentrations of 1, 5 and 10 ng/mL. MTT assay showed that TGF-β1 contributed to cell proliferation in a concentration-dependent manner (Figure 4A). Meanwhile, we observed that the colony formation ability, migration and invasion were promoted by TGF-β1 treatment for 48 h (Figure 4B,C). Immunoblot analysis uncovered that TGF-β1 treatment induced a decrease in E-cadherin expression level and increases in expression levels of Snail, N-cadherin and Vimentin (Figure 4D,E). Then, we further found that TGF-β1 treatment for 48 h augmented XIST expression and depressed miR-137 expression (Figure 4F). Taken together, these data elucidated that TGF-β1 stimulates XIST expression but inhibits miR-137 expression.
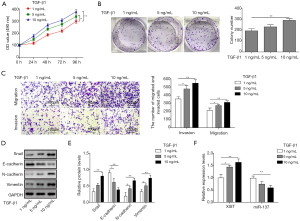
EDIL3 regulates proliferation, migration, invasion and EMT in gastric cancer cells through modulating TGF-β1 activity
In addition, AGS cells overexpressed EDIL3 treated with TGF-β1 inhibitor. MTT analysis showed that EDIL3 upregulation accelerated AGS cell growth, migration and invasion, which could be inhibited by TGF-β1 inhibitor (Figure 5A,B). As shown in Figure 5C, EDIL3 overexpression elevated expression levels of EDIL3, Snail, N-cadherin and Vimentin but reduced expression level of E-cadherin, which also could be reversed by the TGF-β1 inhibitor treatment. Accordingly, these data indicated that EDIL3 regulates proliferation, migration, invasion and EMT in AGS cells through the TGF-β1 signal pathway.
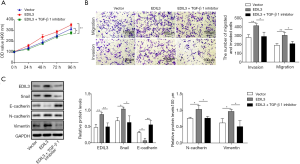
EDIL3 regulates proliferation, migration, invasion and EMT in AGS cells through modulating TGF-β1/XIST/miR-137 feedback loop
Our data show that TGF-β1 activates XIST which inhibits miR-137 expression to increase the activity of EDIL3, while EDIL3 regulates the expression of TGF-β1. These findings lead us to propose a hypothesis of EDIL3/TGF-β1/XIST/miR-137 feedback loop. Firstly, to further confirm that TGF-β1/XIST/miR-137 axis is regulated by EDIL3, we investigated whether recombinant TGF-β1 treatment, XIST overexpression can rescue the inhibitory role of EDIL3 knockdown on cell proliferation, migration, invasion and EMT in AGCs cells. The results showed that TGF-β1 treatment, XIST overexpression can rescue the inhibitory role of EDIL3 knockdown on cell proliferation, migration, invasion and EMT in AGCs cells as shown in Figure 6A-C. Secondly, to confirm EDIL3 is the downstream of miR-137, besides the analysis of the 3'-UTR and dual-luciferase reporter assay, we made them more convincing if EDIL3 overexpression rescues cell proliferation, migration, invasion and EMT in AGCs cells inhibited by miR-137 mimics. The results showed that EDIL3 overexpression rescued cell proliferation, migration, invasion and EMT in AGCs cells inhibited by miR-137 mimics (Figure 6D,F). We conclude that EDIL3 can function as a tumor activator and form a positive feedback loop with TGF-β1 expression. Once TGF-β1 is activated, XIST expression is increased. The more XIST is expressed, the more its target genes of miR-137 are suppressed. The decrease in miR-137 expression permits an increase in EDIL3 activity. Increased EDIL3 enhances TGF-β1/XIST axis production and then inhibits miR-137 expression, completing a positive feedback loop.
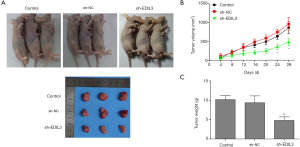
EDIL3 knockdown inhibits tumor growth in nude mice
In a previous in vitro study, we reported that EDIL3 promoted cell proliferation in two gastric cancer cell lines, including AGS and MKN-45. In this study, we also used the experimental AGS gastric cancer xenograft model to verify the inhibitory effect of EDIL3 knockdown. EDIL3 knockdown or control AGS cells (5×106) were injected into nude mice, we found that tumors produced by the control cells displayed fast growth and tumor volume was remarkably smaller in the sh-EDIL3 group than that in the control group (Figure 7A,B, P<0.05). Similarly, the mice in the sh-EDIL3 group showed a significant reduction in tumor weight, as compared with those in the control group (Figure 7C, P<0.01), supporting the notion that the reduced EDIL3 may inhibit growth in gastric cancer cells.
Discussion
Even though multiple therapeutic strategies have been applied in gastric cancer, it remains an important cancer with rather high mortality internationally and complex and ambiguous risk factors (17). In human gastric cancer, it could be transferred into other tissues including liver, which augmented the tumor development and reduced survival time and life quality of patients (18). In addition, the EMT is associated with the cancer cell metastasis (19,20). Xiang et al. claimed that gastric cancer metastasis was regulated by C-X-C chemokine receptor type 4 (CXCR4) and CXCR2 (21). Chen et al. found that sparc/osteonectin, cwcv and kazal-like domain proteoglycan 1 (SPOCK1) could control the metastasis of gastric cancer by Slug-induced EMT (22). In view of these facts, it can be found that the mechanisms of gastric cancer metastasis and development are complex. Moreover, the more potential mechanism underlying the gastric cancer metastasis and development need to be explored.
EDIL3 is a pro-cancer factor by facilitating cancer cell migration (23). miRNAs, as non-coding RNAs, are common regulators on biocharacteristics in tumors. EDIL3 has been reported to be regulated by several miRNAs such as miR-137 and miR-375-3p (13,24). Moreover, growing evidences indicated that miR-137 was expressed lowly in gastric cancer and modulated the tumor development by various downstream signaling targets (25,26). In terms of miR-137 regulation, XIST exerts a direct binding to miR-137 for functioning on neuropathic pain, blood-tumor barrier permeability, and cancer cell growth and EMT (16,27,28). Similarly, we elaborated that the binding relationship of miR-137 and EDIL3 or XIST was solidly validated by the dual-luciferase reporter detection. EMT is involved in the participation of Snail, N-cadherin, E-cadherin as well as Vimentin. In this study, it was demonstrated that EDIL3 and XIST possessed positive effects on EMT, in addition to gastric cancer cell growth, migration and invasion; while miR-137 exerted negative effects on it.
TGF-β1 is a common cytokine and promotes fibrogenesis and cell proliferation. It is well-known that there is TGF-β1 expression with high level in cancer cells. Ananiev et al. reported that the expression of TGF-β1 in the gastric cancer patients was associated with shorter survival time and rapid progression (29). Generally, TGF-β1 can induce EMT in various cancer cells, such as breast cancer cells, hepatocellular carcinoma and neuroblastoma cells (30-32). TGF-β1 positively regulates the expression of XIST (14). XIST works as an endogenous sponge via directly binding to miR-137 (28). Furthermore, as illustrated above, there is a binding correlation between miR-137 and EDIL3. Importantly, EDIL3 could exert a regulation on TGF-β1 pathway (11). Taken together, it can be inferred that the EDIL3/TGF-β1/XIST/miR-137 feedback loop may be a critical process for carcinoma development. Expectedly, in our study, adding exogenous TGF-β1 accelerated the development of gastric cancer cells by stimulating their proliferation, migration, invasion and even EMT, in accompany with elevated expression of XIST and decreased expression of miR-137. The promotion of the gastric cancer biological characteristics triggered by EDIL3 upregulation was reserved by TGF-β1 inhibition.
In conclusion, we have proved it forcefully that EDIL3 possesses a regulatory action on gastric cancer cell migration, invasion and EMT, and the mechanism of its modulation on gastric cancer biological characteristics is involved in the regulation of TGF-β1/XIST/miR-137 feedback loop. Meanwhile, our findings in this research further reveals a novel regulatory pathway of metastasis and development of the gastric tumor, and the components of the EDIL3/TGF-β1/XIST/miR-137 molecular pathway can be used as prognostic indicators of the gastric cancer, and even be developed as drug targets for the treatment of the gastric carcinoma.
Acknowledgments
Funding: This work is supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2967b
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2967b
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-19-2967b
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2967b). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee board of Guizhou Provincial People’s Hospital and informed consent was taken from all the patients. Animals experiments were performed by the Committee on the Ethics of Animal Experiments of Guizhou Provincial People’s Hospital, in compliance with National Institutes of Health guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700-13. [Crossref] [PubMed]
- Akhondi-Meybodi M, Ghane M, Akhondi-Meybodi S, et al. Five-year Survival Rate for Gastric Cancer in Yazd Province, Central Iran, from 2001 to 2008. Middle East J Dig Dis 2017;9:39-48. [Crossref] [PubMed]
- Li D, Li D, Song G, et al. Cancer survival in Cixian of China, 2003-2013: a population-based study. Cancer Med 2018;7:1537-45. [Crossref] [PubMed]
- Chaffer CL, San Juan BP, Lim E, et al. EMT, cell plasticity and metastasis. Cancer Metastasis Rev 2016;35:645-54. [Crossref] [PubMed]
- Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015;6:10697-711. [Crossref] [PubMed]
- Wang G, Fu Y, Liu G, et al. miR-218 Inhibits Proliferation, Migration, and EMT of Gastric Cancer Cells by Targeting WASF3. Oncol Res 2017;25:355-64. [Crossref] [PubMed]
- Yoon JH, Choi WS, Kim O, et al. Gastrokine 1 inhibits gastric cancer cell migration and invasion by downregulating RhoA expression. Gastric Cancer. 2017;20:274-285. [Crossref] [PubMed]
- Niu X, Chang W, Liu R, et al. mRNA and protein expression of the angiogenesis-related genes EDIL3, AMOT and ECM1 in mesenchymal stem cells in psoriatic dermis. Clin Exp Dermatol 2016;41:533-40. [Crossref] [PubMed]
- Jiang SH, Wang Y, Yang JY, et al. Overexpressed EDIL3 predicts poor prognosis and promotes anchorage-independent tumor growth in human pancreatic cancer. Oncotarget 2016;7:4226-40. [Crossref] [PubMed]
- Sun JC, Liang XT, Pan K, et al. High expression level of EDIL3 in HCC predicts poor prognosis of HCC patients. World J Gastroenterol 2010;16:4611-5. [Crossref] [PubMed]
- Zhang R, Wei YH, Zhao CY, et al. EDIL3 depletion suppress epithelial-mesenchymal transition of lens epithelial cells via transforming growth factor β pathway. Int J Ophthalmol 2018;11:18-24. [PubMed]
- Sripathy S, Leko V, Adrianse RL, et al. Screen for reactivation of MeCP2 on the inactive X chromosome identifies the BMP/TGF-β superfamily as a regulator of XIST expression. Proc Natl Acad Sci U S A 2017;114:1619-24. [Crossref] [PubMed]
- Xia H, Chen J, Shi M, et al. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol 2015;63:863-73. [Crossref] [PubMed]
- Zhang Q, Chen B, Liu P, et al. XIST promotes gastric cancer (GC) progression through TGF-β1 via targeting miR-185. J Cell Biochem 2018;119:2787-96. [Crossref] [PubMed]
- Ma L, Zhou Y, Luo X, et al. Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancer. Oncotarget 2017;8:4125-35. [Crossref] [PubMed]
- Yu H, Xue Y, Wang P, et al. Knockdown of long non-coding RNA XIST increases blood-tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis 2017;6:e303. [Crossref] [PubMed]
- Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer 2014;101:E1-12. [Crossref] [PubMed]
- Liu YJ, Wang GC, Wan XB, et al. Surgical resection for gastric cancer patients with liver metastasis. Zhonghua Zhong Liu Za Zhi 2017;39:532-5. [PubMed]
- Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 2016;43:7-13. [Crossref] [PubMed]
- Jolly MK, Ware KE, Gilja S, et al. EMT and MET: necessary or permissive for metastasis? Mol Oncol 2017;11:755-69. [Crossref] [PubMed]
- Xiang Z, Zhou ZJ, Xia GK, et al. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene 2017;36:5122-33. [Crossref] [PubMed]
- Chen D, Zhou H, Liu G, et al. SPOCK1 promotes the invasion and metastasis of gastric cancer through Slug-induced epithelial-mesenchymal transition. J Cell Mol Med 2018;22:797-807. [Crossref] [PubMed]
- Beckham CJ, Olsen J, Yin PN, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol 2014;192:583-92. [Crossref] [PubMed]
- Cen WN, Pang JS, Huang JC, et al. The expression and biological information analysis of miR-375-3p in head and neck squamous cell carcinoma based on 1825 samples from GEO, TCGA, and peer-reviewed publications. Pathol Res Pract 2018;214:1835-47. [Crossref] [PubMed]
- Chen Q, Chen X, Zhang M, et al. miR-137 is frequently down-regulated in gastric cancer and is a negative regulator of Cdc42. Dig Dis Sci 2011;56:2009-16. [Crossref] [PubMed]
- Cheng Y, Li Y, Liu D, et al. miR-137 effects on gastric carcinogenesis are mediated by targeting Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett 2014;588:3274-81. [Crossref] [PubMed]
- Zhao Y, Li S, Xia N, et al. Effects of XIST/miR-137 axis on neuropathic pain by targeting TNFAIP1 in a rat model. J Cell Physiol 2018;233:4307-16. [Crossref] [PubMed]
- Wang X, Zhang G, Cheng Z, et al. Knockdown of LncRNA-XIST Suppresses Proliferation and TGF-β1-Induced EMT in NSCLC Through the Notch-1 Pathway by Regulation of miR-137. Genet Test Mol Biomarkers 2018;22:333-342. [Crossref] [PubMed]
- Ananiev J, Manolova I, Aleksandrova E, et al. Impact of TGF-β1 expression and -509C>T polymorphism in the TGF-β1 gene on the progression and survival of gastric cancer. Pol J Pathol 2017;68:234-40. [Crossref] [PubMed]
- Ma F, Li W, Liu C, et al. MiR-23a promotes TGF-β1-induced EMT and tumor metastasis in breast cancer cells by directly targeting CDH1 and activating Wnt/β-catenin signaling. Oncotarget 2017;8:69538-50. [Crossref] [PubMed]
- Qin G, Luo M, Chen J, et al. Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett 2016;374:85-95. [Crossref] [PubMed]
- Shao JB, Gao ZM, Huang WY, et al. The mechanism of epithelial- mesenchymal transition induced by TGF-β1 in neuroblastoma cells. Int J Oncol 2017;50:1623-33. [Crossref] [PubMed]


