
Expression of YAP in endometrial carcinoma tissues and its effect on epithelial to mesenchymal transition
Introduction
Annually and globally, endometrial carcinoma (EC), a common gynecological epithelial malignancy, victimizes over 200,000 females, a population that makes up 6% of all females with malignancies (1). EC has complex pathogenesis co-regulated by genetic and environmental factors. Among them, genetic mutation plays a vital role. The mutation and polymorphism of protooncogenes, cancer suppressor genes, estrogen-related genes, estrogen/progesterone receptor genes can increase the risk of EC (2). The cancer cells invade the myometrium in the early stages of EC, but the recurrence, mortality, and prognosis of patients are mainly decided by the cancer cells’ direct extension/penetration, lymphatic metastasis, or migration to distal organs (3). Therefore, genetically clarifying the regulatory mechanism hiding behind EC development may supply a therapeutic target for EC.
Human yes-associated protein (YAP) was first found as researchers were screening the genetic mutants in fruit flies in 1994, located in11q22 amplicon (4). YAP has no DNA-binding sites, but its structural domains and specific amino acid sequences (5) enable YAP to interact with various proteins (like transcriptional factors in the TEAD family, cyclin E) in the regulation of signaling pathways. Recent studies have evaluated the aberrantly high expression of YAP that facilitates the development of pancreatic ductal adenocarcinoma, serous ovarian carcinoma, pulmonary small cell carcinoma, urinary bladder carcinoma (6-9). Wang et al. found that YAP promoted the malignancy of endometrial cancer cells via regulation of IL-6 and IL-11 (10). In addition, Konno et al. confirmed that ASPP2 suppression promoted malignancy via LSR and YAP in human endometrial cancer (11). However, Yuan et al. (12). discovered that the YAP gene was deficiently expressed in invasive breast carcinoma, showing its role as a suppressor, which was consequently confirmed by the immunohistochemical analysis performed by Jaramillo-Rodríguez et al. (13).
YAP acts as a powerful regulator in epithelial to mesenchymal transition (EMT), even cancerous invasion and migration (14). During EMT, epithelial cellulose their original morphological characteristics and differentiate into mesenchymal cells that have already gained a spindle shape and motility. Biochemically, epithelial cell markers are expressed and transformed into mesenchymal markers, like E-cadherin and its counterpart N-cadherin. Meanwhile, Vimentin is also abnormally expressed. The present study verified that YAP regulates the EMT and other biological processes involved in EC through promoting the proliferation, invasion, and migration of EC cells. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-3155).
Methods
Samples
A total of 52 EC tissue samples and 20 normal endometrial tissue samples were prepared. The EC tissues were obtained from patients (ages 38–55 years, mean 45.3 years) having undergone comprehensive staging laparotomy at the First Affiliated Hospital of Anhui Medical University. The first treatment the patients received was surgery: extra fascial abdominal radical/extensive hysterectomy, bilateral salpingo-oophorectomy, pelvic lymphadenectomy. The patients did not receive chemotherapy, radiotherapy, and hormonotherapy before the surgery. The ECs were staged according to the staging system released by the International Federation of Gynecology and Obstetrics (IFGO): stage I, 22 cases; stage II, 20 cases, stage III, 10 cases. Of these 52 cases, 43 showed no lymphatic metastasis. The EC was histologically graded according to the WHO classification system [2003]: grade 1, 21 cases; grade 2, 7 cases; grade 3, 24 cases. The patients aged 38–55 years (average of 45.3 years).
A normal control group was constructed with 20 normal endometrial tissue samples provided by the Hysteroscopy Department of the hospital. These tissues were obtained from the proliferative-phase endometrium of outpatient-hysteroscopy-treated patients (ages 38–54 years, mean 43.4 years) for subserous hysteromyoma (12 cases) or lodged contraceptive rings (8 cases). Intraoperative and postoperative pathological examination confirms the normality of all the tissues.
The ethics committee approved the sampling procedures of the Affiliated Hospital of Anhui Medical University. All the patients presented their informed consent. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013).
Cell culture and plasmid transfection
HEC-1-A and Ishikawa cells (BeNa Culture Collection, China) were allowed to thaw and then cultured with DMEM medium (Gibico, USA) containing 10% fetal bovine serum in an incubator (37 °C, 5% CO2). Cell passage was allowed once every 3–4 days. From the information on YAP (NM_1130145) in GenBank, three YAP-specific shRNA sequences (GV102) and Shanghai Genechem Co. Ltd constructed one eukaryotic expression vector (Shanghai, China).
Lipofectamine 2000 was used to transfect YAP-shRNAI, YAP-shRNAII, and YAP-shRNAIII into Ishikawa cells. The normal control group and YAP-shRNA transfection group were set up. GV230/YAP was transfected into HEC-1-A cells. The normal control group and GV230 transfection group were set up. A standard protocol was performed in triplicate.
RT-PCR
Total RNA was extracted from the cells using the DP430 kits and the tissues using DP431 kits provided by (Tiangen Biotech Co. Ltd., Beijing, China). The ultraviolet spectrophotometer was used for RNA-seq analysis. The RNA was reverse transcribed into cDNA using reverse transcription kits (Thermo Fish, USA). The RT-PCR kit was used to detect the expression of the YAP gene, E-cadherin, and Vimentin. (Glyceraldehydes phosphate dehydrogenase) GAPDH was used as an internal control. Primers of YAP gene (forward: 5'-TGACCCTCGTTTTGCCATGA-3; reverse: 5'-GTTGCTGCTGGTTGGAGTTG-3'), GAPDH (forward: 5'-CTTCTTTTGCGTCGCCAGCC-3'; reverse: 5'-GCCCAATACGACCAAATCCGT-3'); E-cadherin (forward: 5'-TCGCTTACACCATCCTCAGC-3'; reverse: 5'-GGAAACTCTCTCGGTCCAGC-3'); N-cadherin (forward: 5'-AACAGCAACGACGGGTTAGT-3'; reverse: 5'-CAGACACGGTTGCAGTTGAC-3'); Vimentin (forward: 5'-AGGCGAGGAGAGCAGGATTT-3'; reverse: 5'-AGTGGGTATCAACCAGAGGGA-3’).
RT-PCR was performed (95 °C for 30 s, 95 °C for 5 s, 60 °C for 34 s; 40 cycles). The gene expression was calculated with the ∆∆Ct method in each group. Relative quantification=2-∆∆Ct. A standard protocol was performed in triplicate for all primers.
Western blot (WB)
Total protein was extracted with radio-immunoprecipitation assay (RIPA), quantified with bicinchoninic acid (BCA), injected into the well (50 mcg/well) on a sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) gel. After electrophoretic separation, the protein was moved to the PVDF membrane. Then, the protein was stored at room temperature for 1.5 hours, incubated with primary antibodies at 4 °C overnight, and with secondary antibodies at room temperature for 1 hour. At last, enhanced electrochemiluminescence (ECL) plus developer was applied to develop X-ray films. Quantity One software was used to analyze the grey value (pixel density) of each protein band. The expression level of the protein of interest = grey value of protein of interest/grey value of GAPDH of interest.
Cell wound healing assay
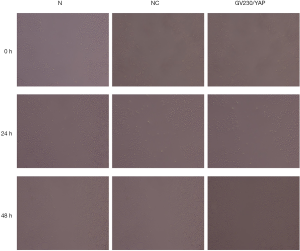
The cells were seeded into a 6-well dish. A 10 µm razor blade in a vertical line was used on the dish surface to create the scratch. Three eye-piece fields were randomly selected to capture images at 0 hours, 24 hours, and 48 hours during the assay. The migration rate of cells was measured. The migration rate = (scratch width at 0 hours − width of the scratch at 24 hours/48 hours)/scratch width at 0 hours.
CCK8 cell proliferation assay
The cells were seeded in a 96-well plate (2×105 cells/well), put in an incubator for 24 hours at 37 °C, and 5% CO2. The same standard protocol was duplicated eight times. According to the CCK8 kit (Bestbio Co. Ltd., Shanghai, China), 10 µL of CCK8 solution was injected into each well and incubated for 2 hours at 37 °C and 5% CO2. Absorbance was detected at a wavelength of 450 nm. The rate of proliferation = (ODcontrol group − ODuntreated group) − (ODtreatment group − ODuntreated group)/(ODcontrol − ODuntreated group) ×100%.
Statistical analysis
PSS 13.0 was used to analyze data presented as mean ± SD. The enumerated data were described with percent. Chi-square was performed for comparison. Measurement data were presented as mean ± SD. One-way ANOVA was performed to compare multiple groups; the LSD t-test was done for the between-group comparison. P<0.01 was considered significantly different.
Results
Expression of YAP in EC tissues
The expression level of YAP in EC tissues was significantly higher than that in normal tissues in the proliferative phase (4.998±1.173 vs. 0.841±0.222; P<0.01) (Figures 1,2). As shown by the pathological examination, the mRNA and protein expression levels of YAP increased in all three grades of tissues (G1, G2, G3). As shown by the between-group comparison, the mRNA expression level of YAP shows between-group differences (P<0.01). Considering the clinical stages of EC, the YAP expression on both levels significantly increased in three stages (stage I, stage II, stage III), but no between-group difference showed up (P>0.01). The expression of YAP on both levels in the lymphatic metastasis group and none lymphatic metastasis group was quantified as 5.582±0.633 vs. 3.582±0.533, 0.826±0.346 vs. 0.396±0.157 (P<0.01) (Table 1).
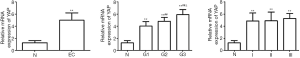
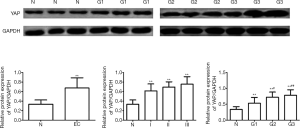
Table 1
| Stages and grades | Groups | Cases | YAP protein expression | YAP mRNA expression | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | ||||||
| Pathologic grades | G1 | 21 | 0.534±0.184 | 10.68 | 0.00 | 4.021±0.766 | 30.660 | 0.000 | |
| G2 | 7 | 0.720±0.162 | 4.839±0.602 | ||||||
| G3 | 24 | 0.781±0.172 | 5.900±0.840 | ||||||
| FIGO stages | I | 22 | 0.615±0.242 | 1.718 | 0.19 | 4.868±1.318 | 0.341 | 0.713 | |
| II | 20 | 0.696±0.109 | 4.912±1.426 | ||||||
| III | 10 | 0.755±0.251 | 5.275±0.832 | ||||||
| Lymphatic metastasis | Present | 9 | 0.826±0.346 | 0.00 | 5.582±0.633 | 0.000 | |||
| Absent | 43 | 0.396±0.157 | 3.582±0.533 | ||||||
The mRNA expression level of YAP peaked in G3 and showed a difference between groups of different grades (P<0.01). Nevertheless, its expression on both levels showed no grade- or stage-related difference (P=0.713, 0.19). YAP, yes-associated protein; EC, endometrial carcinoma.
Proliferation, invasion, and migration of EC cells promoted by PAP in vitro
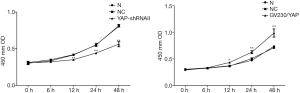
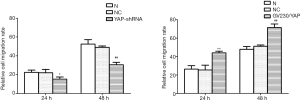
YAP interference and overexpression vectors were constructed. YAP-shRNAII with high interference efficacy was selected for functional assays (Figure 3). Ishikawa and HEC-1A cell lines were transfected (transfection efficiency >75%). Overexpressed YAP was stably transfected into HEC-1A cell lines (Figure 4). As shown by the results of CCK8 assay, compared to the normal control group, at 12 after downregulating YAP expression, the proliferative ability of Ishikawa cells began to drop. However, at 24 hours after upregulating YAP expression, this ability of HEC-1A cells began to rise (Figure 5). As shown by the cell wound healing assay, compared to the normal control group, at 24 hours and 48 hours after downregulating YAP expression, the migratory ability of Ishikawa cells dropped by 7.23%±2.39% and 22.04%±5.92%, respectively; simultaneously pointing after upregulating YAP expression, this ability of HEC-1A cells increased by 7.23%±2.39% and 22.04%±5.92%, respectively. These findings suggested that the YAP gene could increase the proliferation, invasion, and migration of EC cells (Figures 6-8).
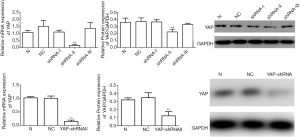
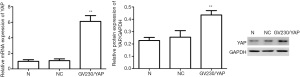

EMT accelerated by YAP in EC cell lines
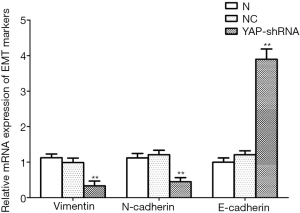
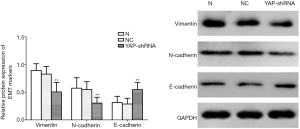
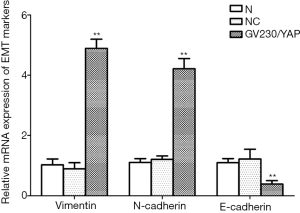
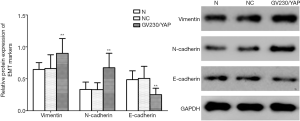
Given the performances of YAP mentioned above, we detected the mRNA and protein expression of EMT markers after YAP interference and overexpression. As shown by the RT-PCR and WB results, compared to the normal control group, both mRNA and protein expression levels of E-cadherin increased obviously (P<0.01), but all those of N-cadherin and Vimentin fell obviously in the YAP-shRNAII transfection group (P<0.01) (Figures 9,10). Besides, an inversed trend was observed in the GV230/YAP overexpression group (Figures 11,12). These findings suggested that YAP could promote the invasion and migration of EC through accelerating EMT.
Discussion
Previously known as a protein playing a central role in the Hippo signaling pathway, YAP can promote the growth, inhibit the apoptosis, abolish the contact inhibition, exacerbate the malignancy of cancer cells (15). In this experiment, we found the mRNA and protein expression levels of YPA rose in EC tissues, indicating the cancer-promoting role of YAP in EC. Tsujiura et al. (16) investigated 120 cases of type I and 30 cases of type II endometrioid adenocarcinoma, finding that the YAP gene was expressed in both nuclei cytoplasm. Among type I cases, 55.0% (66/120) showed high YAP expression in nuclei and 68.3% (82/120) in the cytoplasm; among type II cases, these two percentages climbed to 90.0% (27/30) and 63.3% (19/30). This finding is consistent with ours. Tsujiura also verified the tight association between YAP expression level and the cell differentiation, pathologic grade, lymphovascular space invasion, recurrence, and metastasis of type I EC, but this association did not occur to type II EC.
Our statistical results proved that the YAP expression level was linked with pathologic grade and metastasis, not with the clinical stage. Opinions conflict with each about the role of YAP (promotor or suppressor) in cancer development. Recent studies (4,17,18) hypothesize this conflict may arise because YAP bears various phosphorylation sites and can bind various activators. YAP has no DNA-binding sites, so the transcriptional co-activators binding YAP maneuvers its functions. It acts as a cancer promoter once bound to TEAD, and a cancer suppressor once bound to P73/P63. Nevertheless, the hiding mechanism is still to be dugout.
The present experiment verified that high YAP expression promoted the development of EC despite its pathologic grade and lymphatic metastasis. That high YAP expression can benefit tumorigenesis, and EMT has been validated by researching hepatocellular carcinoma, oral squamous cell carcinoma, colon cancer, and ovarian cancer (19-23). In this in vitro experiment, downregulating YAP expression inhibited the proliferation, invasion, and migration of Ishikawa cells, a process that curbed the progression of EMT. Together, upregulating YAP expression powered the proliferation, invasion, and migration of HEC-1A cells, a process that fueled the progression of EMT. These suggest that YAP can regulate the invasion and migration of EC cells, and the EMT or other biological processes in EC.
We demonstrated that up-regulation and down-regulation of YAP could increase and decrease the proliferation and differentiation of EC cells. Although further mechanisms and in vivo evidence have not been fully demonstrated, our data suggest targeted inhibition. YAP may be a potential treatment for EC patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-3155
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-3155
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-3155). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Research Ethics Committee approved the present study of the First Affiliated Hospital of Anhui Medical University. All the patients presented their informed consent. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67-73. [Crossref] [PubMed]
- Fan W, Jiang H, Chen H, et al. Esophageal metastasis from endometrial adenocarcinoma: a case report and literature review. Transl Cancer Res 2018;7:1178-83. [Crossref]
- Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 1994;9:2145-52. [PubMed]
- Chen L, Chan SW, Zhang X, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev 2010;24:290-300. [Crossref] [PubMed]
- Kapoor A, Yao W, Ying H, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 2014;158:185-97. [Crossref] [PubMed]
- Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol 2008;39:1582-9. [Crossref] [PubMed]
- Wang Y, Dong Q, Zhang Q, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 2010;101:1279-85. [Crossref] [PubMed]
- Liu JY, Li YH, Lin HX, et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer 2013;13:349. [Crossref] [PubMed]
- Wang J, Song T, Zhou S, et al. YAP promotes the malignancy of endometrial cancer cells via regulation of IL-6 and IL-11. Mol Med 2019;25:32. [Crossref] [PubMed]
- Konno T, Kohno T, Okada T, et al. ASPP2 suppression promotes malignancy via LSR and YAP in human endometrial cancer. Histochem Cell Biol 2020;154:197-213. [Crossref] [PubMed]
- Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ 2008;15:1752-9. [Crossref] [PubMed]
- Jaramillo-Rodríguez Y, Cerda-Flores RM, Ruiz-Ramos R, et al. YAP expression in normal and neoplastic breast tissue: an immunohistochemical study. Arch Med Res 2014;45:223-8. [Crossref] [PubMed]
- Yu SJ, Hu JY, Kuang XY, et al. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin Cancer Res 2013;19:1389-99. [Crossref] [PubMed]
- Liu M, Zhao S, Lin Q, et al. YAP regulates the expression of Hoxa1 and Hoxc13 in mouse and human oral and skin epithelial tissues. Mol Cell Biol 2015;35:1449-61. [Crossref] [PubMed]
- Tsujiura M, Mazack V, Sudol M, et al. Yes-associated protein (YAP) modulates oncogenic features and radiation sensitivity in endometrial cancer. PLoS One 2014;9:e100974. [Crossref] [PubMed]
- Kaneko K, Ito M, Naoe Y, et al. Integrin αv in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun 2014;447:352-7. [Crossref] [PubMed]
- Tomlinson V, Gudmundsdottir K, Luong P, et al. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis 2010;1:e29. [Crossref] [PubMed]
- Li H, Wang S, Wang G, et al. Yes-associated protein expression is a predictive marker for recurrence of hepatocellular carcinoma after liver transplantation. Dig Surg 2014;31:468-78. [Crossref] [PubMed]
- Malakou LS, Gargalionis AN, Piperi C, et al. Molecular mechanisms of mechanotransduction in psoriasis. Ann Transl Med. 2018;6:245. [Crossref] [PubMed]
- Shao DD, Xue W, Krall EB, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 2014;158:171-84. [Crossref] [PubMed]
- He C, Lv X, Hua G, et al. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene 2015;34:6040-54. [Crossref] [PubMed]
- Xia Y, Chang T, Wang Y, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One 2014;9:e91770. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


