
A case of brain glioma progression: surgical resection and post-operation nursing care
Glioma, arising from neuroepithelial tissues, is the most common brain tumor and account for about 50–60% of all primary brain tumors (1,2). According to the statistics of Shanghai Huashan Hospital from 1951 to 2011, glioma mobility was about 41.13%. Glioma is histologically classified as malignant brain tumors [from World Health Organization (WHO) grade I to IV] (3). Among them, WHO grade I and grade II astrocytoma and oligodendroglioma belong to low grade glioma, while WHO grade III and grade IV astrocytoma and oligodendroglioma are high grade gliomas. The standard therapy strategies for glioma is surgical resection, accompanied by chemotherapy and radiotherapy (4,5). The prognosis of low grade glioma are relatively well, however, high grade gliomas are aggressive with poor survival period, especially glioblastoma multiforme (GBM).
Here, we reported the first brain glioma case in our department since our hospital foundation, and we resected the tumor under the assistance of experts from Shanghai Huashan Hospital (including the post-operation nursing care). The cooperation was very meaningful in the history of our hospital, and is very important for the academic construction of our neurosurgery department in the future.
Case presentation
A 62-year-old male patient suffered from progressive headache and weakness of his right limbs for about 3 months. Brain magnetic resonance imaging (MRI) scan at our hospital showed left frontal lesion (low grade glioma is highly suspected, Figure 1). However, the patient refused our operation suggestion, and underwent radiotherapy at another hospital. Three months after radiotherapy, the patient family members complained to us that the patient’s consciousness was progressively confused. At admission, the patient was fatigue and difficult to communicate. His bilateral pupils were round and equal with diameters of 2.5 mm, and light reflex was regular. Rightside muscle strength was grade III, and Babinski sign was positive.
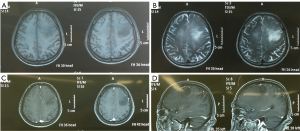
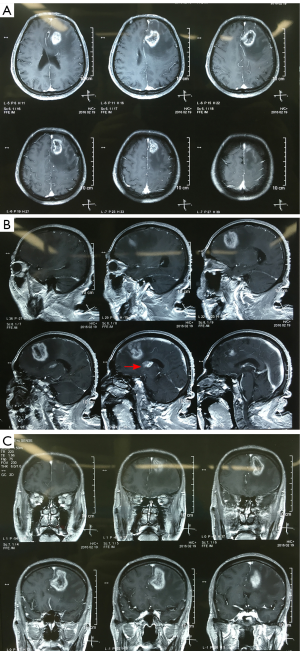
After admission, we performed brain MRI scan for the patient. Massive lesion was found at left frontal lobe and basal ganglia area with irregular enhancement in contrast MRI (Figure 2). High grade glioma is highly suspected, and we advised surgical removal for the patient.
Surgical resection
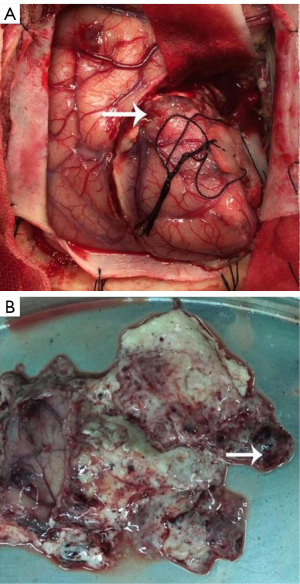
Left frontal craniotomy was performed to remove the tumor after patient and his family members consent. During the operation, we found the tumor boundary was unclear, and separated the tumor along its adjacent brain edema area. The tumor was gray and soft with moderate blood supply (Figure 3A). A little necrosis tissues were found inside the tumor (Figure 3B). Finally, the left frontal tumor was totally removed, however, tumors located at left basal ganglia area and thalamus weren’t resected in consideration of preservation for limb movement function, which was requested by the patient’s family members. After surgery, the patient could raise his right limbs and speak a little.
Pathological findings
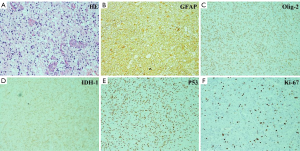
Histological findings revealed anaplastic astrocytoma with partial GBM features (a little necrosis was found inside the tumor) (3). The principal histopathological features are diffusely infiltrating astrocytoma with increased cellularity, distinct nuclear atypia and mitotic activity (Figure 4A). For immunohistochemical staining, the tumor cells were massively positive for glial fibrillary acidic protein (GFAP, Figure 4B), and partial positive for oligodendrocyte cells (Figure 4C). For genetics, high frequency of IDH-1 (Figure 4D) and P53 (Figure 4E) mutations was observed as well. The Ki-67/MIB-1 represented cellular proliferation and it was about 30% in the tumor (Figure 4F).
Post-operation nursing care
Post-operation nursing care is very important for patients with cerebral glioma. After operation, the patient was carefully cared by nurses at our department under the instruction from Shanghai Huashan Hospital. Daily fluid input and output volume was recorded and kept steady, especially for elderly patient, in order to keep the internal environment in balance, including the electrolyte. Consciousness status should be observed, because post-operation brain edema maybe very severe and cause consciousness disorder. Hence, mannitol infusion should be delivered daily. Epilepsy was one of the most common complications, thus sodium valproate was administered for such patients. Moreover, tongue-spatula should be prepared at bedside in order to prevent tongue bite injury, and fall-down should be avoided by protective restriction. Fever was also very common generally caused by intracranial infection after operation or pneumonia, especially in elderly patient. Thus, turn-over and backslapping was necessary for patients if pneumonia happens. Nutrition deficiency was observed in this patient, and nutrition support was implemented for him by our nutrition department. Later, the patient was gradually recovered, and he could raise his right limbs and speak a little at discharge. And chemotherapy was recommended for the patient for the next period of treatment.
Discussion
Gliomas are the most common primary tumors affecting the adult central nervous system (CNS), accounting for about 50–60% of all primary brain tumors, characterized by high morbidity and mortality as well as high recurrence rate (6,7). Studies found that gliomas are presumed to arise from mature glia or neural stem cells (NSCs) and diffusely infiltrate the surrounding tissues (8-10), making surgical resection very difficult. Survival of gliomas depends on the grades of malignancy. The 2007 WHO defines four classes (I, II, III, IV) for glioma on the basis of their morphological features, proliferation behavior and genetic mutations. The most lethal is grade IV glioblastoma (GBM), with a 5-year survival rate of less than 10% due to difficulties in complete resection and the low sensitivity to radiotherapy and chemotherapy (11).
In the paper, we reported the first brain glioma case in our department since our hospital foundation, and we treated the patient under the assistance of experts from Shanghai Huashan Hospital. The patient refused surgical resection at initial diagnosis and received radiotherapy; however, tumor progression was happened after the radiotherapy. High grade glioma was highly suspected from the brain MRI images (Figure 2) compared to the images before radiotherapy (Figure 1). We used left frontal approach to resect the tumor. The tumor was soft and without clear border (Figure 3). Finally, the left frontal tumor was totally removed; however, the left basal ganglia area tumor wasn’t resected in consideration of preservation for limb movement function, which was requested by the patient’s family members.
The post-operation pathological identification revealed anaplastic astrocytoma with partial GBM features. Anaplastic astrocytoma may arise from diffuse astrocytoma WHO grade II and has an inherent tendency to undergo progression to glioblastoma. Anaplastic astrocytoma is histologically characterized by nuclear atypia, increased cellularity and significant proliferative activity (Figure 4). The tumor cells were massively positive for glial fibrillary acidic protein. For genetics (12-14), high frequency of IDH-1 and P53 mutations was observed. The cellular proliferation indexed by Ki-67 was about 30% in the tumor.
Nursing care is very important for glioma patients after operation (15). Because the most common post-operative complications, such as brain edema, seizure attack and fever may occur. Daily fluid input and output volume should be recorded by nurses and kept in balance. Consciousness status should be observed, because post-operation brain edema maybe very severe and cause consciousness disorder or coma. Tongue-spatula should be prepared at bedside in case of tongue bite injury caused by seizure attack, and protective restrictions should be applied to avoid fall-down. In some patients, fever may happen caused by infection or pneumonia, which is very troublesome for elderly patient, thus, turn-over and backslapping were necessary. Moreover, nutrition support should be paid attention to old patients as well. We recommended further chemotherapy for the patient after discharge.
Conclusions
In our paper, we reported the first brain glioma case in our department since our hospital foundation, and we resected the tumor under the assistance of experts from Shanghai Huashan Hospital (including the post-operation nursing care). From the whole treatment point of view, it was a great successful case of cooperation. The cooperation is very meaningful in the history of our hospital, and is very important for the academic construction of our neurosurgery department in the future.
Acknowledgments
We are very grateful to Dr. Haixia Li from the Department of Pathology at Shanghai Huashan Hospital for her kind assistance in pathological consultation.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.05.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cavaleri JM, Monaco EA 3rd. New Insights Into the Genomic Alterations in Glioma Progression. Neurosurgery 2016;78:N11-3.
- Zhang RR, Pointer KB, Kuo JS. New Molecular Insights and Potential Therapies for Diffuse Intrinsic Pontine Glioma. Neurosurgery 2015;77:N13-4.
- Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Nervous System. Lyon: IARC, 2007;30-49.
- Han SJ, Englot DJ, Birk H, et al. Impact of Timing of Concurrent Chemoradiation for Newly Diagnosed Glioblastoma: A Critical Review of Current Evidence. Neurosurgery 2015;62:160-5. [Crossref] [PubMed]
- Reulen HJ, Poepperl G, Goetz C, et al. Long-term outcome of patients with WHO Grade III and IV gliomas treated by fractionated intracavitary radioimmunotherapy. J Neurosurg 2015;123:760-70. [Crossref] [PubMed]
- Osswald M, Jung E, Sahm F, et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015;528:93-8. [PubMed]
- Xu R, Fang XH, Zhong P. Myosin VI contributes to malignant proliferation of human glioma cells. Korean J Physiol Pharmacol 2016;20:139-45. [Crossref] [PubMed]
- Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 2010;10:319-31. [Crossref] [PubMed]
- Friedmann-Morvinski D, Bushong EA, Ke E, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 2012;338:1080-4. [Crossref] [PubMed]
- Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 2012;149:36-47. [Crossref] [PubMed]
- Ceccarelli M, Barthel FP, Malta TM, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016;164:550-63. [Crossref] [PubMed]
- Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462-77. [Crossref] [PubMed]
- Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013;340:626-30. [Crossref] [PubMed]
- Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014;512:324-7. [Crossref] [PubMed]
- Lang L, Yan Z, Tang H. Home visits in brain tumor patient: how nurse and family members cooperate in tumor patient's family self-care. Chin J Cancer Res 2013;25:729-34. [PubMed]


