
CD147 handles lipid: a new role for anti-cancer target
The future of anti-cancer therapy is promising as immunotherapy strategies have been revolutionary in treatment of a number of cancers. However, some organs such as the liver are characterised by immunosuppression of CD4+ and CD8+ activity, necessitating alternative strategies for cancers in such tissues. This occurs at least partly through aberrant expression of alpha-fetoprotein that in turn dampens immune activation (1). Hepatocellular carcinoma (HCC) is the second leading cause of death from cancer worldwide (2). Complementary strategies to CD4+ and CD8+ T cell-mediated immunotherapies that specifically target HCC are needed for the future of effective and comprehensive cancer therapy. On the short-list for a molecular target for such a therapy is CD147/EMMPRIN.
CD147 is a transmembrane vesicular and cell-surface glycoprotein with two N-terminal extracellular-oriented immunoglobulin domains. CD147 was discovered as a factor produced from tumour cells that induced matrix metalloproteinases (MMPs) and collagenase activity from fibroblasts (3), thus gaining the moniker extracellular matrix metalloproteinase inducer (EMMPRIN) (4). While various tissues have detectable expression of CD147, a high percentage of malignant cancers overexpress the protein, including HCC (5,6). The use of 131I labelled CD147 specific humanized antibodies has been approved by the Chinese Food and Drug Administration under the drug name licartin or metuximab, and is entering Chinese clinics (7). Niu et al. were able to show that immunologically increasing the concentration of the radioisotope at tumor sites results in diminished tumor size and extended survival for treated rabbits relative to controls (8). Furthermore, individuals with advanced HCC who received licartin post-liver transplantation had less recurrence of HCC compared to placebo one year post-transplantation (26.7% vs. 57.1%) (9).
Despite advances in using CD147 as a tumor targeting molecule, the biology of CD147 remains complex, with multiple regulating factors and pluripotent activities (Figure 1) (4,6). The alias EMMPRIN indicates CD147’s role in inducing fibroblasts to secrete MMPs, namely MMP-1, MMP-2, MMP-3, MMP-9, MT1-MMP, and MT2-MMP (4,10). The stimulatory effect of these proteases could play a role in activating invasion and metastasis, one of the hallmarks of cancer (13). CD147 can homo-oligomerize and bind a number of binding partners such as cyclophilin A and monocarboxylate transporters (MCTs). Soluble cyclophilin A promotes migration and proliferation of multiple myeloma cells by binding to CD147, and plays a role in chemotaxis of myeloma cells from the blood to the bone marrow (14). CD147 complexes with MCTs to symport lactic acid and protons out of the cell, acidic products of the fermentative glycolysis produced by rapidly proliferating tumour cells (11). In the context of tumor growth, this function of CD147 allows rapid proliferation and metabolism by glycolysis, the “Warburg effect”, without generating an acidic intracellular milieu. Over-expression of CD147 correlates with higher glucose uptake, lactate production, and diminished p53 expression relative to CD147 silenced cells (12,15).
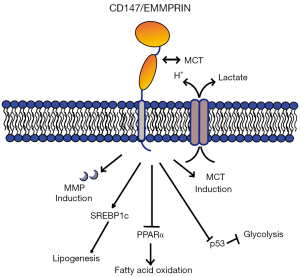
Rapidly proliferating tumor tissue requires energy consumption yielded by glycolysis and lactic acid fermentation, and also needs production of cellular components for cell division, including a high demand for phospholipid synthesis. Since CD147 appears to act as a control switch for cells to enter anaerobic glycolysis, could this have knock-on effects into lipid metabolism? Li and colleagues utilized 4 public RNA-seq datasets of HCC tissues and found a correlation between CD147 expression and increased expression of genes involved in fatty acid metabolism (7). The authors then used two different HCC cell lines to down-regulate or knock out CD147 expression, along with restoring CD147 expression in the knocked-out line, to observe differences in fatty acid metabolism. CD147 expressing cells not only had higher levels of phospholipids but also had high triglyceride levels; similar to the lipid droplet accumulation observed in cancers previously (16). CD147 expression correlated with the lipogenesis promoting transcription factor SREBP1c, as well as downstream genes encoding lipogenic enzymes. Lipogenesis in HCC has been associated with the AKT-mTORC1 pathway (17), and analysis of these engineered cells showed that CD147 expression aligned with activation of this pathway. Similar lines of evidence showed correlations between diminished fatty acid oxidation genes through suppression of transcription factor PPARα through MAPK signalling. The authors then implanted the CD147 knocked-out HCC cell lines, the cells with ectopically restored SREBP1c expression, or the cells with silenced PPARα expression into the livers of nude mice. Both increased lipogenesis and diminished fatty acid oxidation separately could restore oncogenesis despite CD147 knockout, indicating that fatty acid metabolism is a key instrument in which CD147 is oncogenic.
CD147 is overexpressed in a number of tumor types and is a therapeutic target for directed immune-radiotherapy against HCC. Investigation of CD147 has revealed a number of different functions for the protein yet with ambiguous direct effects. Li et al. used genetic techniques to show that CD147 exerts tumorigenic activity by both upregulating lipogenesis and down-regulating fatty acid oxidation, thus meeting the phospholipid requirements for cell proliferation. The multiple roles played by CD147 appear related in that they all encourage rapid proliferation. These roles include inducing MMPs to restructure the extracellular matrix to make space for new cells, eschewing lactate to encourage rapid glycolysis and energy production, and now promoting lipogenesis to build membranes of daughter cells. The mystery that remains to be clarified is how does CD147, a surface transmembrane protein, play all of these roles. Since CD147 is a transmembrane protein with characteristic N-terminal immunoglobulin domains, the effect is likely through signalling but there is a paucity of data describing intrinsic signalling motifs in CD147 (4). There is much cross-talk between glucose and lipid metabolism pathways and it may be difficult to precisely define the mechanism involving CD147’s role in these processes. Investigations into this hub of so many oncogenic effects need to occur in order to improve our understanding of tumor development and treatment.
Acknowledgments
Funding: TF Baumert acknowledges grant support by the European Union (ERC-2008-AdG-HEPCENT, ERC-AdG-2014-HEPCIR, FP7 HepaMab, and Interreg IV FEDER-Hepato-Regio-Net 2012), the Agence Nationale de Recherche sur le SIDA (ANRS) and the Direction Générale de l'Offre de Soins (A12027MS).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor An-Qiang Wang, MD (Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ritter M, Ali MY, Grimm CF, et al. Immunoregulation of dendritic and T cells by alpha-fetoprotein in patients with hepatocellular carcinoma. J Hepatol 2004;41:999-1007. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Kataoka H, DeCastro R, Zucker S, et al. Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res 1993;53:3154-8. [PubMed]
- Grass GD, Toole BP. How, with whom and when: an overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity. Biosci Rep 2015;36:e00283 [Crossref] [PubMed]
- Riethdorf S, Reimers N, Assmann V, et al. High incidence of EMMPRIN expression in human tumors. Int J Cancer 2006;119:1800-10. [Crossref] [PubMed]
- Xiong L, Edwards CK 3rd, Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int J Mol Sci 2014;15:17411-41. [Crossref] [PubMed]
- Li J, Huang Q, Long X, et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol 2015;63:1378-89. [Crossref] [PubMed]
- Niu H, Wang R, Cheng J, et al. Treatment of (131)I-labeled anti-CD147 monoclonal antibody in VX2 carcinoma-induced liver tumors. Oncol Rep 2013;30:246-52. [PubMed]
- Xu J, Shen ZY, Chen XG, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007;45:269-76. [Crossref] [PubMed]
- Guo H, Zucker S, Gordon MK, et al. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem 1997;272:24-7. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Zhu D, Wang Z, Zhao JJ, et al. The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat Med 2015;21:572-80. [Crossref] [PubMed]
- Le Floch R, Chiche J, Marchiq I, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A 2011;108:16663-8. [Crossref] [PubMed]
- Huang Q, Li J, Xing J, et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol 2014;61:859-66. [Crossref] [PubMed]
- Schneiderhan W, Scheler M, Holzmann KH, et al. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut 2009;58:1391-8. [Crossref] [PubMed]
- Currie E, Schulze A, Zechner R, et al. Cellular fatty acid metabolism and cancer. Cell Metab 2013;18:153-61. [Crossref] [PubMed]
- Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011;140:1071-83. [Crossref] [PubMed]


