
Harnessing protein kinase A activation to induce mesenchymal-epithelial programs to eliminate chemoresistant, tumor-initiating breast cancer cells
Introduction
Metastatic dissemination of primary tumors remains the most significant predictor of clinical outcomes of cancer patients, as well as the most lethal characteristic of human malignancies, including those that arise in the breast (1,2). Breast tumors are highly heterogeneous and comprised of numerous subclones that emanate from an array of genetic and epigenetic variants that coalesce in enhancing the fitness of disseminated cells upon their colonization of distant vital organs (3). Moreover, disseminated tumor cells (DTCs) are typically the culprits underlying clinical relapse in breast cancer patients, a process that transpires through the ability of DTCs to acquire tumor-initiating/stem-like and chemoresistant phenotypes, traits that cement DTCs as one of the foremost barriers to eradicating metastatic disease. The molecular mechanisms responsible for metastatic relapse and chemoresistance are multipartite and remain to be fully elucidated; however, recent findings implicate epithelial-mesenchymal transition (EMT) programs as a major driver that (I) induces metastatic cells to detach and egress from the primary tumor; and (II) enables DTCs to surmount the cellular and genotoxic stressors imposed by foreign microenvironments and cytotoxic therapies (4-7). In the most general sense, EMT programs reflect an organized transdifferentiation process whereby polarized epithelial cells shed their immotile behaviors in favor of newly acquired fibroblastoid-like phenotypes characterized by increased invasive and migratory capabilities that compel indolent carcinoma in situ lesions to become highly aggressive invasive lesions (4,7-9).
It is interesting to note that the relevance of EMT programs to breast cancer metastasis was originally questioned by pathologists due to the paradoxical and unexpected finding that newly established secondary tumor lesions were in many respects histologically indistinguishable from their pre-EMT primary tumor sites. This perplexing behavior exhibited by DTCs is now recognized to reflect the induction of mesenchymal-epithelial transition (MET) programs, which phenotypically and morphologically reverse the plastic activities of EMT, thereby enabling nonproliferative post-EMT cells to reactivate proliferative states necessary for the formation of overt metastases. Indeed, the importance of MET programs to drive metastatic outgrowth is highlighted by the fact that perpetual mesenchymal (e.g., post-EMT) states actually hinder the ability of DTCs to colonize distant tissues, a process that is circumvented by their acquisition of epithelial states elicited by MET programs (10,11). Metastatic colonization is also aided by breast cancer stem cells (CSCs), whose capacity for self-renewal heavily influences disease recurrence and, consequently, the clinical outcomes of breast cancer (12,13). Moreover, the activation of EMT programs readily produces a distinct population of cells that possess phenotypes and behaviors reminiscent of those observed in normal and malignant stem cells (14-17), indicating that EMT contributes mightily to the tumor-initiating capacity (i.e., “stemness”) of malignant cells. The parallels between EMT programs and “stem-like” states coalesce on another important malignant behavior, namely the acquisition of chemoresistant phenotypes. Indeed, CSCs have long been recognized to possess an inherent resistance to conventional chemotherapies, as these tumor-initiating cells (TICs) are often quiescent and naturally immune to cytotoxic agents (18). Likewise, CSCs also maintain gene expression profiles that ensure for their survival when confronted with chemotherapies, doing so in part by upregulating their expression of drug efflux pumps, and by sustaining their ability to cope with drug-induced oxidative stress (18,19). Importantly, recent evidence indicates that the ability of EMT programs to impart chemoresistant phenotypes upon CSCs (20-22) may in fact be dissociated from those operant in conferring metastasis-promoting activities in human malignancies (23,24), suggesting that the targeted inactivation of EMT programs may offer new inroads capable of restoring breast cancer patient response to conventional chemotherapies.
Protein kinase A (PKA): getting reacquainted with an old friend
The consistent and repeated finding in nature that post-EMT carcinoma cells display mesenchymal, chemoresistant, and tumor-initiating phenotypes in response to a host of disparate extrinsic and intrinsic mediators suggests two important points: (I) the molecular underpinnings coupled to cell plasticity pathways exhibit significant overlap and functional redundancy; and (II) discovering and disrupting these vital molecular underpinnings may offer new “differentiation-based” strategies capable of alleviating post-EMT cells, thereby improving the clinical course of patients with metastatic disease. Accordingly, a provocative study recently published by Pattabiraman and colleagues (25) implicates the activation of PKA as an essential signaling nexus operant in driving mammary epithelial cell differentiation and fate. Historically, and owing to the discovery of its phosphorylation and activation of phosphorylase kinase, the stimulation of PKA by cyclic adenosine monophosphate (cAMP) represented the first signaling cascade and module identified and characterized, and presently, more information is known regarding the molecular, structural, and physiological functions of this protein kinase relative to all others housed within the human kinome (26). Indeed, PKA activation readily oversees the capacity of cells to either proliferate or differentiate, doing so by altering their genetic and epigenetic landscapes, and by remodeling their actin cytoskeletons. Importantly, these cellular events enable PKA to govern a diverse array of physiological processes, including embryogenesis and development, cardiac and neuronal function, steroidogenesis, and immune homeostasis, as well as the responses of tissues to a host of hormones, neurotransmitters, and peptides (27).
The ubiquitous nature of PKA in regulating cell and tissue homeostasis suggests that dysregulated signaling by this protein kinase may contribute to the development of a variety of human diseases, including cancer, wherein PKA influences malignant phenotypes in a paradoxical manner. For instance, overexpression of the RI regulatory subunit of PKA is frequently observed in human cancers and plays an essential role in driving cell cycle progression, and in eliciting chemoresistance phenotypes. Conversely, and typically dependent upon microenvironmental cues, PKA can also adopt a tumor suppressive role in cells whose PKA activity is predominantly governed by the RII regulatory subunit (28-31). At present, the best defined contribution of PKA signaling to neoplastic transformation is found in endocrine-associated tumors, including those arising in the kidney, pituitary, thyroid, and testis, where elevated activation of PKA is highly associated with tumor aggression (32). Along these lines, PKA activation has also been linked to the induction of EMT programs due to its ability to promote cytoskeletal remodeling and migratory behaviors in malignant cells (33,34); it also serves as a critical mediator of EMT programs activated by hypoxia (35), and as a potential driver of chemoresistance in breast cancer cells (36). Consistent with its dichotomous roles during tumorigenesis, PKA activation has also been linked to the induction of MET programs and a return to more differentiated phenotypes in certain cancers (37), an activity Pattabiraman and colleagues (25) attempted to exploit as a novel therapy in the treatment of metastatic breast cancers.
PKA induces MET and suppresses TIC tumorigenicity
The genetic and morphologic plasticity of CSCs/TICs remains a significant barrier to eradicating these drivers of disease in clinical breast cancer settings. Classically, attempts to interdict EMT programs have relied on strategies designed to (I) inhibit the initial induction of these transdifferentiation events; or (II) inactivate proteins expressed specifically in post-EMT cells that are essential for their survival. Another longstanding, but largely untested strategy [see (38)] posits that identifying the molecular mechanisms coupled to MET programs may represent an innovative strategy to impede the primary tumor metastasis (10,11). Moreover, MET-based strategies are in many respects analogous to those aimed at inducing cellular differentiation, thus potentially having the added bonus of resensitizing post-MET cells to anticancer agents. With this idea in mind, Pattabiraman et al. screened a 400 compound library to identify agents capable of restoring E-cadherin expression in human NAMEC8 (N8) mammary epithelial cells, which derive from HMLE cells and display all the classical features of post-EMT cells, including (I) a prominent mesenchymal morphology and corresponding gene expression profile; (II) a heightened migratory and invasive behavior; (III) an enhanced CSC phenotype and elevated propensity to form mammospheres; and (IV) an increased insensitivity to anticancer agents, such as doxorubicin and paclitaxel. In doing so, the authors identified the adenylate cyclase activator, forskolin, as a potent stimulator of E-cadherin expression, and subsequently, as a reliable inducer of MET programs that diminished the malignant and stem-like features of N8 cells, as well as enhanced their sensitivity to various cytotoxic agents (e.g., doxorubicin and paclitaxel). Identifying the cAMP effector responsible for driving MET programs initiated by forskolin was accomplished via several complementary analyses. Indeed, administration of cholera toxin (Ctx), which elevates intracellular cAMP levels by ADP-ribosylating and constitutively-activating Gαs, wholly recapitulated the MET-inducing properties of forskolin, as did the addition of the potent PKA activator and stable cAMP analog, 8-Br-cAMP (8-bromoadenosine 3',5'-cyclic monophosphate). Importantly, administration of 8-CPT-2Me-cAMP, which selectively activates exchange protein activated by cAMP (EPACs) as compared to PKA, failed to impact the mesenchymal characteristic of N8 cells, suggesting that PKA activation drives MET programs in breast cancer cells. Accordingly, rendering N8 cells deficient in PKA expression abrogated the ability of Ctx to induce MET programs, while engineering these cells to express a constitutively-active PKA mutant was sufficient in eliciting MET programs independent of increased cAMP levels. The functional significance of the cAMP:PKA signaling axis in driving MET programs was not restricted solely to N8 cells, as similar MET-based responses were readily observed in additional breast, lung, pancreas, and ovarian carcinoma cell lines when treated with Ctx or forskolin. Mechanistically, the research team identified the histone demethylase PHF2 as the primary phosphoprotein targeted by PKA as it induces MET programs in N8 cells. Indeed, upon its phosphorylation and activation by PKA, PHF2 localizes to distinct genomic loci where it relieves repressive histone methylation marks necessary to induce MET programs and the expression of epithelial gene signatures (Figure 1). As a means to extend their findings to preclinical settings, the authors undertook two xenograft platforms in mice. First, Ctx treatment of metastatic N8-Ras cells not only suppressed their ability to colonize the lungs of mice, but also inhibited their tumor-initiating capacity when implanted at limiting dilutions into mice. Second, utilizing a doxycycline-inducible system to drive the expression of constitutively-active PKA in developing N8-Ras tumors, the authors once again strongly suppressed the growth and tumor-initiating properties of these breast cancer cells. Collectively, this intriguing study identified a novel cAMP:PKA:PHF2 signaling axis that targets the epigenome as a means to stimulate MET programs in breast cancer cells, thereby suppressing their metastatic and tumor-initiating behaviors.
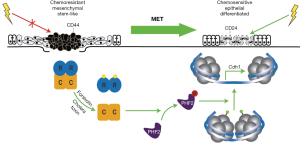
Future directions for MET-directed therapies: friends or foes
Drugs directed at G protein-coupled receptors (GPCRs) represent the most abundant and successful class of pharmaceutical agents developed to treat human diseases (39); however, similarly effective and robust targeting of the ubiquitous cAMP/PKA signaling axis remains elusive due to the unacceptably high toxicities and off-target activities associated with the administration of these agents (40,41). Presumably these difficulties precluded Pattabiraman et al. (25) from undertaking a more direct preclinical therapy model capable of pharmacologically activating PKA in DTCs, particularly after these cells take up residence and begin to recur at distant locales. This line of research is essential to undertake for several important reasons. First, ~50% of women already harbor DTCs in their bone marrow and vital organs when initially diagnosed with breast cancer, while ~62% of breast cancer deaths occur 5–20 years after initial diagnosis (42-44). These data imply that DTCs play pivotal roles in the majority of breast cancer-associated mortalities, thus cementing DTCs as one of the most clinically relevant targets in all of oncology. Second, current dogma states that MET programs compel DTCs to reactivate proliferative programs necessary for efficient metastatic outgrowth, and as such, one might predict that inducing MET programs in DTCs could in fact enhance their ability to recur and expand within metastatic niches. Interestingly, Pattabiraman and colleagues (25) only analyzed the impact MET programs exert on the dissemination and tumor-initiating capacity of breast cancer cells within primary tumor lesions, sites clearly expected to be highly sensitive to and impacted by PKA-mediated induction of MET programs. In fact, the growth of post-MET tumors (i.e., more epithelioid) in mice was not significantly different from that of their pre-MET counterparts (i.e., more mesenchymal), suggesting that the induction of MET programs falls short in resetting the differentiation status of cells harboring malignant genomes. Thus, future studies are clearly warranted to assess the impact of PKA activation on the proliferative capacity of breast cancer micro- and macrometastases, and on their relative sensitivities to chemotherapeutic agents in preclinical models, studies that were noticeably absent in the work by Pattabiraman et al. (25). Equally intriguing is the need to investigate the potential effectiveness of PKA activation in neoadjuvant settings as an innovative means to suppress surgery-induced cancer metastasis.
Although Pattabiraman and colleagues (25) clearly established PKA as a mediator of MET programs and reduced breast tumor growth, it should be noted that similar increases in cAMP/PKA signaling are typically associated with cell cycle progression, a finding that supports the inhibition of this signaling axis in therapeutic settings (45). The discrepancy between these two approaches may be explained by the fact that the pro-proliferative signals engendered by PKA apply predominantly to normal differentiated cells, not to their malignant dedifferentiated counterparts. This theory is supported by the observation that tonic intracellular cAMP levels in malignant cells are often dramatically lower than those measured in their normal counterparts (45,46). It is interesting to note that while Pattabiraman et al. (25) employed Ctx as a means to elevate cAMP levels and stimulate PKA, clinical approaches to activate PKA signaling networks have traditionally relied on the use of phosphodiesterase (PDE) inhibitors, which increase cAMP concentrations by preventing its degradation. At present, the clinical utility of PDE inhibitors in suppressing tumor development and progression is still being evaluated; however, PDE inhibitors have been shown to prevent the growth and induce the death of a variety of malignant cells (47-49), including those of the breast (50,51). Thus, future studies should investigate the impact of PDE inhibitors to drive PKA-mediated activation of PHF2 and MET programs, and consequently to inhibit the tumor-initiating capacity, metastatic ability, and chemosensitivity of breast tumors and their DTCs.
Finally, the precise interplay and relationship between EMT and CSCs remains a complicated and intriguing aspect of cancer biology. Indeed, although EMT programs have been shown to precede and drive the expansion of CSCs (14-17), a recent study demonstrated that EMT programs can suppress the “stemness” of prostate and bladder cancer cell lines, whose tumor-initiating capacity and metastatic activity could be restored by MET programs (52). Similarly, the EMT activator, Prrx, is a positive predictor of clinical outcome that cooperates with the EMT-associated transcription factor Twist1 to suppress the “stemness” and proliferation of various breast cancer cell lines (10). Although the molecular and cellular mechanisms underlying these discrepancies remain to be elucidated, it is tempting to speculate that differences in the cAMP/PKA signaling dictate the outcome of EMT-MET programs in human cancers. For instance heightened expression of the RI regulatory subunit relative to that of its RII counterpart predicts for disease recurrence and poor overall patient survival in breast cancer patients (53). Thus, future studies need to determine how the expression patterns of the RI and RII regulatory subunits are impacted by EMT-MET programs, as well as how these events influence the tumor-initiating capacity of epithelial-like and mesenchymal-like breast cancer cells. Ultimately, answering these and the other aforementioned questions will provide the necessary foundation to develop more effective therapies against breast cancer DTCs.
Acknowledgments
Funding: This study was supported by the National Institutes of Health to AJ Gooding (T32GM007250 and CA203233) and WP Schiemann (CA129359, CA177069, and CA194518).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vanharanta S, Massagué J. Origins of metastatic traits. Cancer Cell 2013;24:410-21. [Crossref] [PubMed]
- Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016;529:298-306. [Crossref] [PubMed]
- Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer 2015;15:473-83. [Crossref] [PubMed]
- Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia 2010;15:169-90. [Crossref] [PubMed]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741-51. [Crossref] [PubMed]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 2010;15:117-34. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol 2009;5:1145-68. [Crossref] [PubMed]
- Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res 2012;347:85-101. [Crossref] [PubMed]
- Ocaña OH, Córcoles R, Fabra A, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 2012;22:709-24. [Crossref] [PubMed]
- Tsai JH, Donaher JL, Murphy DA, et al. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012;22:725-36. [Crossref] [PubMed]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell 2014;14:275-91. [Crossref] [PubMed]
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013;501:346-54. [Crossref] [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [Crossref] [PubMed]
- Morel AP, Lièvre M. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008;3:e2888 [Crossref] [PubMed]
- Ye X, Tam WL, Shibue T, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 2015;525:256-60. [Crossref] [PubMed]
- Guo W, Keckesova Z, Donaher JL, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 2012;148:1015-28. [Crossref] [PubMed]
- Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015;6:10697-711. [Crossref] [PubMed]
- Ahmed N, Abubaker K, Findlay J, et al. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Curr Cancer Drug Targets 2010;10:268-78. [Crossref] [PubMed]
- Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A ;2009:13820-5. [PubMed]
- Wang X, Ling MT, Guan XY, et al. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene 2004;23:474-82. [Crossref] [PubMed]
- Li QQ, Xu JD, Wang WJ, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res 2009;15:2657-65. [Crossref] [PubMed]
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525-30. [Crossref] [PubMed]
- Fischer K, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistanceNature 2015;527:472-6.
- Pattabiraman DR, Bierie B, Kober KI, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 2016;351:aad3680 [Crossref] [PubMed]
- Roskoski R Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol Res 2015;100:1-23. [Crossref] [PubMed]
- Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 2004;84:137-67. [Crossref] [PubMed]
- Stratakis CA. cAMP/PKA signaling defects in tumors: genetics and tissue-specific pluripotential cell-derived lesions in human and mouse. Mol Cell Endocrinol 2013;371:208-20. [Crossref] [PubMed]
- Tortora G, Ciardiello F. Protein kinase A as target for novel integrated strategies of cancer therapy. Ann N Y Acad Sci 2002;968:139-47. [Crossref] [PubMed]
- Cho-Chung YS, Nesterova MV. Tumor reversion: protein kinase A isozyme switching. Ann N Y Acad Sci 2005;1058:76-86. [Crossref] [PubMed]
- Insel PA, Zhang L, Murray F, et al. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 2012;204:277-87. [Crossref] [PubMed]
- Yu B, Ragazzon B, Rizk-Rabin M, et al. Protein kinase A alterations in endocrine tumors. Horm Metab Res 2012;44:741-8. [Crossref] [PubMed]
- Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta 2004;1692:159-74.
- Jiang P, Enomoto A, Takahashi M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett 2009;284:122-30. [Crossref] [PubMed]
- Shaikh D, Zhou Q, Chen T, et al. cAMP-dependent protein kinase is essential for hypoxia-mediated epithelial-mesenchymal transition, migration, and invasion in lung cancer cells. Cell Signal 2012;24:2396-406. [Crossref] [PubMed]
- de Leeuw R, Flach K, Bentin Toaldo C, et al. PKA phosphorylation redirects ERα to promoters of a unique gene set to induce tamoxifen resistance. Oncogene 2013;32:3543-51. [Crossref] [PubMed]
- Nadella KS, Jones GN, Trimboli A, et al. Targeted deletion of Prkar1a reveals a role for protein kinase A in mesenchymal-to-epithelial transition. Cancer Res 2008;68:2671-7. [Crossref] [PubMed]
- Allington TM, Schiemann WP. The Cain and Abl of epithelial-mesenchymal transition and transforming growth factor-β in mammary epithelial cells. Cells Tissues Organs 2011;193:98-113. [Crossref] [PubMed]
- Bradley SJ, Tobin AB. Design of Next-Generation G Protein-Coupled Receptor Drugs: Linking Novel Pharmacology and In Vivo Animal Models. Annu Rev Pharmacol Toxicol 2016;56:535-59. [Crossref] [PubMed]
- Propper DJ, Saunders MP, Salisbury AJ, et al. Phase I study of the novel cyclic AMP (cAMP) analogue 8-chloro-cAMP in patients with cancer: toxicity, hormonal, and immunological effects. Clin Cancer Res 1999;5:1682-9. [PubMed]
- Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 2008;1:re4. [Crossref] [PubMed]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004;4:448-56. [Crossref] [PubMed]
- Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev 2011;21:42-9. [Crossref] [PubMed]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014;14:611-22. [Crossref] [PubMed]
- Dumont JE, Jauniaux JC, Roger PP. The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci 1989;14:67-71. [Crossref] [PubMed]
- Drees M, Zimmermann R, Eisenbrand G. 3',5'-Cyclic nucleotide phosphodiesterase in tumor cells as potential target for tumor growth inhibition. Cancer Res 1993;53:3058-61. [PubMed]
- Marko D, Romanakis K, Zank H, et al. Induction of apoptosis by an inhibitor of cAMP-specific PDE in malignant murine carcinoma cells overexpressing PDE activity in comparison to their nonmalignant counterparts. Cell Biochem Biophys 1998;28:75-101. [Crossref] [PubMed]
- Zhang L, Murray F, Zahno A, et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2008;105:19532-7. [Crossref] [PubMed]
- Murata K, Sudo T, Kameyama M, et al. Cyclic AMP specific phosphodiesterase activity and colon cancer cell motility. Clin Exp Metastasis 2000;18:599-604. [Crossref] [PubMed]
- Mahdian D, Shafiee-Nick R, Mousavi SH. Different effects of adenylyl cyclase activators and phosphodiesterases inhibitors on cervical cancer (HeLa) and breast cancer (MCF-7) cells proliferation. Toxicol Mech Methods 2014;24:307-14. [Crossref] [PubMed]
- Lin DC, Xu L, Ding LW, et al. Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc Natl Acad Sci U S A 2013;110:6109-14. [Crossref] [PubMed]
- Celià-Terrassa T, Meca-Cortés O, Mateo F, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 2012;122:1849-68. [Crossref] [PubMed]
- Miller WR. Regulatory subunits of PKA and breast cancer. Ann N Y Acad Sci 2002;968:37-48. [Crossref] [PubMed]


