
Bendamustine and rituximab for the treatment of relapsed indolent and mantle cell lymphoma: when timing of a study matters
In a recent issue of The Lancet Oncology, Rummel and colleagues reported the mature results of StiL NHL 2-2003, a multicenter, randomized, open-label, non-inferiority phase 3 trial comparing bendamustine plus rituximab (BR) with fludarabine plus rituximab (FR) in patients with relapsed or refractory (R/R) indolent non-Hodgkin lymphoma (iNHL) or mantle-cell lymphoma (MCL) (1). When this study was conceived, fludarabine-containing regimens were widely used, and rituximab was rapidly being integrated into the chemotherapy regimens for CD20-positive lymphomas (Table 1) (12). This study and others reintroduced bendamustine for the treatment of lymphoid neoplasms after its long clinical abeyance. In fact, the authors reported the results of a simultaneous trial, the StiL NHL 1-2003, demonstrating the superiority of BR as compared to rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone (R-CHOP) in patients with newly diagnosed iNHL or MCL (13).
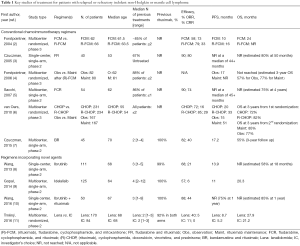
Full table
The StiL NHL 2-2003 included patients with stage II bulky (>7.5 cm), III, or IV iNHL [i.e., grade 1-2 follicular lymphoma (FL), lymphoplasmacytic lymphoma, small lymphocytic lymphoma (SLL), nodular and generalized marginal zone lymphoma (MZL)] and MCL with R/R disease; however, patients refractory to regimens that included rituximab, bendamustine, or purine analogues were excluded. Since rituximab was approved as maintenance for FL during the study, in 2006 the protocol was amended to allow rituximab maintenance (RM) in patients reaching complete remission (CR). The enrollment started on October 8, 2003 and ended on August 5, 2010. In total 230 patients were included: 116 in the BR arm and 114 in the FR. Eleven patients were excluded for not meeting eligibility criteria. Median age was 67 years and the median number of previous treatments was one (mostly CHOP-like regimens). Of note, only 39% of patients in the BR arm and 45% in the FR arm had previously received rituximab.
The overall response rate (ORR) was higher in the BR compared to the FR arm (82% vs. 51%, P<0.0001), as was the CR rate (CRR) (40% vs. 17%, respectively). Of note, the ORR was lower in patients who had previously received rituximab (57%) as compared to those who had not (75%). Furthermore, the BR regimen significantly prolonged the median progression-free survival (PFS) (34.2 vs. 11.7 months, P<0.0001) and overall survival (OS) (109.7 vs. 49.1 months, P=0.012) at a median follow-up of 8 years. Such improvement was confirmed in the MCL, FL and SLL histology. A small group of patients (N=44) received RM and had a significantly longer median PFS as compared to those (N=108) who did not (72.1 vs. 30.4 months, P=0.01). OS was also improved in these patients (not reached vs. 69.7 months, P=0.03). Interestingly, in this unplanned subset analysis, no differences in PFS were observed between those who had originally received BR or FR. Regarding toxicity, no patient discontinued treatment because of drug-related adverse events (AEs). However, 20 patients in both groups needed dose reductions. The most common AEs included infections, myelosuppression, nausea/vomiting, alopecia, and fatigue, without a difference in incidence between the two groups. The overall incidence of serious AEs was also similar.
The study by Rummel and colleagues helps to define the role of BR in the treatment of R/R iNHL or MCL. The study design is solid. The multi-institutional setting and “real-life” eligibility criteria (i.e., leading to a median age of 67 years) render the results generalizable. On the other hand, the lack of central radiology review and open-label treatment administration may partly offset these strengths, as does the per-protocol (rather than intention-to-treat) analysis.
One insurmountable limitation of this trial is its slow accrual rate (it begun in 2003 and completed accrual 7 years later). The study was ultimately published with a median follow-up of 96 months, 13 years after its start. A number of “structural” problems are related to such an extended interval. First, the typical treatment pattern of patients with R/R iNHL or MCL has evolved over the last decade, and thus the population that could be enrolled in a similar trial today would likely differ substantially from the one in this study. Specifically, virtually all patients with CD20-positive lymphoma now receive rituximab as part of their front line therapy (14). The importance of previous rituximab exposure is demonstrated by the fact that the ORR of rituximab-exposed patients was lower than that of rituximab-naïve ones (57% vs. 75%). Thus, in a patient population exposed to rituximab, the PFS may be shorter than reported in this study. Moreover, the subgroup analysis of patients receiving RM after the study was amended (albeit unplanned and thus not adequately powered) showed no PFS difference between BR and FR. Finally, based on the StiL NHL 1–2003 trial (13), the number of patients treated with BR upfront (as opposed to later in the disease course) has significantly increased. The second issue related to the lag time of the study is the selection of FR as a standard comparator. The choice of FR was largely based on the results of a previous phase 2 trial (3). In that study, almost 70% of patients were treatment naïve, and fludarabine was given for 5 consecutive days (vs. 3 in the StiL NHL 2-2003 trial). Thus, the lower-than-expected performance of FR in the Rummel study might have been due in part to a lower fludarabine dose. Lastly, as comorbidities (and not only anagraphic age) can influence treatment tolerability and most patients in StiL NHL 2–2003 had a performance status of 0-1, regimens like fludarabine-mitoxantrone-cyclophosphamide-rituximab (4) or fludarabine-cyclophosphamide-rituximab (5) could have been as tolerated as, and potentially more efficacious than, FR (Table 1). The third issue related to the duration of this trial is the more recent availability of novel agents showing promising results in heavily pretreated, rituximab-exposed patients with R/R iNHL and MCL, including phosphoinositide 3-kinase (PI3K) inhibitors, Bruton tyrosine kinase (BTK) inhibitors, BCL2 inhibitors, and immunomodulators, among others (Table 1).
For example, in a phase 2 study of 125 heavily pretreated iNHL patients (median age 64 years), the PI3K delta inhibitor idelalisib produced an ORR of 57% and a median PFS of 11 months (9). The BTK ibrutinib has also been studied in patients with iNHL or MCL. In a phase 1 trial in patients with various advanced B-cell malignancies, the ORR across dose cohorts was 60%, and the CRR 16%. A response was observed in 7/9 patients with MCL, 11/16 with chronic lymphocytic leukemia/SLL, 6/16 with FL, and 1/4 with MZL. The median PFS was 13.6 months (15). A dedicated study of patients with R/R FL confirmed a somewhat limited ORR after single-agent ibrutinib of 30% (16). However, this improved when the drug was combined with BR (ORR =90%, CRR =50%) (17). A phase 2 study of ibrutinib at a dose of 560 mg daily in R/R MCL patients (N=111, median age 68 years) resulted in an ORR of 68%, including a 21% CRR. The estimated median PFS was 13.9 months (8). In a subsequent phase 3 trial of ibrutinib vs. temsirolimus (N=280) the ORR and CRR were higher with the former (72% and 19% vs. 40% and 1%) and the PFS prolonged (14.6 vs. 6.2 months) (18). Combined with rituximab (N=50, median age 67 years) ibrutinib resulted in an ORR of 88%, including 44% CRR. The 15-month PFS and OS were 69% and 83%, respectively (10). Interestingly, the addition of ibrutinib to BR produced an ORR and CRR of 94% and 76%, respectively (17). Another drug with activity in R/R iNHL and MCL is lenalidomide. In patients with FL, combined lenalidomide and rituximab generated an ORR of 76% and a CRR of 39%. The median time to progression was 2 years (19). In patients with R/R MCL, lenalidomide as a single-agent showed limited activity (11), but when combined with rituximab (N=44), it resulted in an ORR of 57%, and a CRR of 36%, for a median PFS and OS of 11.1 and 24.3 months, respectively. The addition of bendamustine to this doublet was deemed too toxic, and may not be developed further. While the agents described were not available at the time of protocol design, the aforementioned studies exemplify the strikingly different treatment modalities available in the modern-day treatment of R/R iNHL and MCL.
In conclusion, despite the rapidly evolving treatment landscape of iNHL and MCL, the study from Rummel and colleagues provides a rationale to consider BR for the treatment of patients with R/R iNHL or MCL not exposed to this regimen in the front line setting. Given its favorable safety profile, BR has also been considered as a backbone onto which novel agents can be added in selected groups of patients. However, as more insight is gained into the biology of each lymphoma subtype, the use of targeted and molecularly based therapies—without traditional cytotoxic drugs—will predictably expand. Thus, the StiL NHL 2–2003 study is likely to confirm, rather than change, the current clinical practice.
Acknowledgments
Funding: Marco Ruella is supported by grants from the Society for Immunotherapy of Cancer (EMD-Serono Cancer Immunotherapy Clinical Fellowship), the American Association for Cancer Research (Bristol-Myers Squibb Oncology Fellowship in Clinical Cancer Research), the Gabrielle’s Angel Foundation, and the Società Italiana di Ematologia Sperimentale-Associazione Italiana Contro le Leucemie-Linfomi e Mieloma ONLUS.
Footnote
Provenance and Peer Review: This article was commissioned by the Editorial Office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol 2016;17:57-66. [Crossref] [PubMed]
- Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004;104:3064-71. [Crossref] [PubMed]
- Czuczman MS, Koryzna A, Mohr A, et al. Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol 2005;23:694-704. [Crossref] [PubMed]
- Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006;108:4003-8. [Crossref] [PubMed]
- Sacchi S, Pozzi S, Marcheselli R, et al. Rituximab in combination with fludarabine and cyclophosphamide in the treatment of patients with recurrent follicular lymphoma. Cancer 2007;110:121-8. [Crossref] [PubMed]
- van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 2010;28:2853-8. [Crossref] [PubMed]
- Czuczman MS, Goy A, Lamonica D, et al. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann Hematol 2015;94:2025-32. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008-18. [Crossref] [PubMed]
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol 2016;17:48-56. [Crossref] [PubMed]
- Trněný M, Lamy T, Walewski J, et al. Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol 2016;17:319-31. [Crossref] [PubMed]
- Lenz G, Hiddemann W, Dreyling M. The role of fludarabine in the treatment of follicular and mantle cell lymphoma. Cancer 2004;101:883-93. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Zelenetz AD, Gordon LI, Wierda WG, et al. Non-Hodgkin's lymphomas, version 4.2014. J Natl Compr Canc Netw 2014;12:1282-303. [PubMed]
- Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31:88-94. [Crossref] [PubMed]
- Bartlett NL, LaPlant BR, Qi J, et al. Ibrutinib Monotherapy in Relapsed/Refractory Follicular Lymphoma (FL): Preliminary Results of a Phase 2 Consortium (P2C) Trial. Blood 2014;124:800.
- Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 2015;125:242-8. [Crossref] [PubMed]
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770-8. [Crossref] [PubMed]
- Leonard JP, Jung SH, Johnson J, et al. Randomized Trial of Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With Recurrent Follicular Lymphoma: CALGB 50401 (Alliance). J Clin Oncol 2015;33:3635-40. [Crossref] [PubMed]


